* This is Fig 4 of: D Lovley, Microbial energizers: Fuel cells that keep on going. Microbe 1:323, 7/06. It is freely available at: https://www.asmscience.org/content/journal/microbe/10.1128/microbe.1.323.1.

This page was originally developed to provide supplementary materials for a one semester introductory (survey) course in organic and biochemistry.
Links to the home page for the course, to the home page for the site, and contact information are in the navigation bar at the top of this page; they are also at bottom of page.
| Most of the sections below are listed in the order they came up in my Intro Organic/Biochem course. Some items are intended as general; these are at the end of the list. Some comments refer to the textbook by Ouellette, 2/e. | ||
|
Introduction; alkanes + New 7/23/25.
Alkenes (Afrikaans, French, Italian, Portuguese, Russian, Spanish) Aromatic compounds Stereochemistry (Chirality) Alcohols, ethers, sulfur compounds Aldehydes and ketones Carbohydrates + New 9/17/25. Carboxylic acids, etc Lipids (Spanish) |
Amines, amides
Amino acids, proteins, genes Metabolism (supplemental handouts) Spectroscopy Energy resources + New 9/23/25. General (Spanish) | |
| Bottom of page; return links and contact information | ||
| Links to external sites will open in a new window. | ||
| You may find links of interest on my other pages of Internet Resources, either for other specific courses or the chem-miscellaneous, miscellaneous, or introductory pages. All of these are available from the List of pages of Internet resources. | ||
Here are some posts in my Musings newsletter on alkanes. Also included are some miscellaneous posts.
* Added July 23, 2025. Initial studies of an exotic liquid: carbon (July 23, 2025).
* Briefly noted... Methane and life on Enceladus? (July 21, 2021).
* Methane on Mars: a day-night effect (July 12, 2021).
* Do oil dispersants work? Is biodegradability bad? (January 9, 2016).
* Oil in the oceans: made there by bacteria (January 3, 2016).
* Space-based observation of atmospheric methane -- and the Four Corners methane hotspot (December 29, 2014).
* Svalbard is leaking (March 7, 2014).
* Cows on Mars? (November 7, 2012)
* Quiz: NASA's boat (June 29, 2011).
* Ice on fire (August 28, 2009). More posts about methane hydrates are linked there.
* Other Musings posts about methane and natural gas are listed below in the section Energy resources.
For information about using the UC Libraries, including the electronic resources, see the Library Matters page at the web site. That page also includes information about doing searches of the scientific literature, to find articles on a topic that interests you. Major topic areas there include: UC Berkeley library; electronic journals; journal articles; PubMed (Medline) searches; citation searches.
The "General" section of this page has links to sites for a range of organic chem and biochem courses, both at the introductory and regular university levels. Some of the intro sites may be particularly useful for those who would like more materials at the basic level.
Cycloalkanes (cis, trans; axial, equatorial). Dr Phil Bays (St. Mary's College, Indiana) has a good set of practice problems on stereochemistry. The structures are clear, and the questions appropriate. Instant feedback. Although some parts of this are best left until the stereochemistry chemistry, some parts are useful here with cycloalkanes. http://sites.saintmarys.edu/~pbays/Stereochemistry.html. For now, choose the sections that have cycloalkanes in the title. This site is also listed below for stereochemistry.
Do plants make methane?. In 2006 a group reported that plants make methane. A lot of methane -- enough to suggest that plants were major contributors to our global methane budget. This was a surprising finding, since it was inconsistent with our understanding of how methane is made. The paper explored how this might occur, with no clear conclusions; in the absence of any reasonable mechanism, many were skeptical. Since the initial report, others have examined this; results varied, but the emerging consensus was that the initial report was either wrong or exaggerated. This report by Nisbet et al offers a new view. They show that the plants lack any known pathway for making methane. Importantly, they report that plants serve as efficient conduits of methane from the soil, via transpiration. Thus they really do emit methane, but it is not their own production. This proposal makes sense, and accommodates the variable results obtained by others. Is this the last word? Let's see. A news story: Challenge to plant methane link, January 14, 2009. http://news.bbc.co.uk/2/hi/science/nature/7827106.stm. The paper is R E R Nisbet et al, Emission of methane from plants. Proc Royal Soc B 276:1347-1354, 4/09. This paper is listed in the chapter handout for alkanes: Organic/Biochem Chapter handout: Alkanes (pdf file). (All the chapter handouts are available at: Organic/Biochem Chapter handouts.)
New Petroleum-Degrading Bacteria Found at Rancho La Brea Tar Pits in Los Angeles. https://newsroom.ucr.edu/news_item.html?action=page&id=1583. Methane-producing bacteria are responsible for the bubbles seen at the tar pits. News release, May 10, 2007, on the work of J-S Kim & D E Crowley, Univ Calif Riverside.
On a lighter note... the Periodic Table According to Organic Chemists: https://www.uccs.edu/Documents/danderso/orgpt.pdf. From D R Anderson, University of Colorado at Colorado Springs.
My page on Writing, drawing and viewing chemical formulas points to some free programs that may be useful to you both during the course and for your personal use outside the course. These include:
* RasMol, a program for viewing molecular structures. I encourage you to give it a try early in the course, with some small molecules, so you have some experience with it by the time we get to proteins (Ch 15).
* ChemSketch and ISIS/Draw, programs for drawing organic chemical structures. These are useful for drawing structures for word processing documents. Further, the structures you draw can be converted to files that can be viewed as 3D models. Many students find it fun to try this -- drawing your own structures and converting them to 3D models. Both programs will name structures that you draw.
My page Rings: Showing cis/trans and axial/equatorial relationships discusses these particular aspects of cyclohexane rings.
Other pages at my web site that are mentioned with the Alkanes chapter:
- Library Matters;
- Terms: Primary, Secondary, Tertiary, Quaternary;
- Glossary;
- Omitting numbers;
- Books: Suggestions for general science reading.
- The web site also contains a nice periodic table, current through element #118, on the Download page.
- That page also contains ChemFormula, a macro to help you format chemical expressions in Microsoft Word.
Also see the section on Energy resources. Some of the items it contains used to be listed in this section or under Alcohols, ethers, sulfur compounds.
(And alkynes, of course.)
The following two items are related to papers listed in the Further Reading section of the Chapter handout. Both involve work from UC Berkeley.
A UC Berkeley group led by Jay Keasling is working on production of artemisinin, a new type of anti-malarial drug, in microbes (bacteria and yeast). There is an article about this listed in the chapter handout.
* Here is a campus news story on the funding of this project by the Gates Foundation: "QB3 + Gates' millions = a cure? Helped by Microsoft's founder, Jay Keasling and his industry partners hope to create an inexpensive treatment for malaria". January 12, 2005. https://www.berkeley.edu/news/berkeleyan/2005/01/12_keasling.shtml.
* A status report -- a news story, June 4, 2008. "Synthetic yeast to brew up vital malaria drug." https://www.newscientist.com/article/dn14059-synthetic-yeast-to-brew-up-vital-malaria-drug/.
This group of items is also listed for the Biotechnology in the News (BITN) topic Malaria.
A post in my Musings newsletter with another example of metabolic engineering of microbes: A better way to divide 6 by 2: A more efficient way to use sugar (November 10, 2013). This post is also noted below in the section Energy resources.
A Musings post about production of that drug in the plant system: Artemisinin: an improved source? (June 4, 2019).
UCB researchers have shown a role for carotenoids in carrying off excess energy that is absorbed during photosynthesis. Here is a news story on this project in the UCB student newspaper, Daily Cal: The fight against light - Plant molecule functioning in self defense discovered, https://web.archive.org/web/20200810033920/https://archive.dailycal.org/article.php?id=17463. (Now archived.) The work was published: N E Holt et al, Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433, 1/21/05. The paper is free online at: https://www.science.org/doi/abs/10.1126/science.1105833.
Other
Dr Tom Newton at the University of Southern Maine developed an animation showing how a double bond is formed in terms of orbitals. Here is a copy of the figure: Newton's Figure 3, on double bond [link opens in new window]. In particular, note how two p orbitals, one from each bonding C, overlap sideways. This sideways overlap of elongated orbitals is called π (pi) bonding. It provides an easy way to explain why free rotation is not possible around a double bond. ["Regular" bonding is called σ (sigma) bonding.] In effect, this is an animated version of the right hand side of Fig 1.6. (Newton's organic chemistry web site is no longer available.)
Ozonolysis of Alkenes. http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch06/ch6-10.html. We make the general point that the characteristic reaction type of alkenes is addition. However, the oxidation reactions that Ouellette presents in Sect 4.7 certainly do not appear to be additions, given their overall description. These are complex reactions, but the first step really is an addition to the double bond. This page shows the details of the ozonolysis reaction, and shows the primary addition step; look at the malozonide. The page is part of a more comprehensive organic chem site at the Univ of Calgary.
https://pslc.ws/macrog/index.htm. The Macrogalleria site on polymers, at the University of Southern Mississippi: "A cyberwonderland of polymer fun". It is a major educational resource on polymers, and is very readable over a wide range of topics. "AND... for the young and the young at heart, we have the Kid's Macrogalleria that contains much info and much fun, with activities, demos, and even games that are related to polymers." Also available in Afrikaans, French, Italian, Portuguese, Russian, Spanish. (It is also listed on the Internet resources: Chemistry - Miscellaneous page, under Organic.)
World's first 'green' linear polyethylene launched. Press release, Macrh 31, 2008, from the Royal Society of Chemistry about a Brazilian company making ethylene (ethene) -- and then polyethylene -- from bio-ethanol. We note in class that both directions of the simple interconversion between ethanol and ethylene are industrially useful, with the balance typically depending on the price of oil. Brazil has particularly favorable economics of making ethanol, from sugarcane. Now they have extended this: sugarcane --> ethanol by fermentation --> ethylene by chemical dehydration --> polyethylene, as usual. https://www.chemistryworld.com/news/worlds-first-green-linear-polyethylene-launched/3004331.article.
A post in my Musings newsletter on a biological addition polymer made from alkenes: Could a common food plant be used to make rubber? (March 27, 2015).
The 2000 Nobel Prize in Chemistry was awarded to three scientists (including Alan Heeger at UC Santa Barbara) for the discovery of electrically conductive polymers. The polymers are polyacetylenes. You can think of polyacetylene the same way as polyethylene: the polymer is made by addition; the polymer itself has alternating single and double bonds. See the Nobel site: https://www.nobelprize.org/prizes/chemistry/2000/summary/. The article includes an animation of the charge transfer along a polymer molecule, and between a polymer molecule and the dopant.
A post in my Musings newsletter about those polyacetylenes: What if you pulled on the ends of a ladderene? (September 26, 2017).
The 2005 Nobel Prize in Chemistry was awarded to three scientists for the development of alkene metathesis. The idea is to combine smaller alkenes into larger ones, by a process that formally looks something like double replacement (i.e., a rearrangement). See the Nobel site: https://www.nobelprize.org/prizes/chemistry/2005/summary/.
For more about farnesol (p 128, bottom), to the tune of Jingle Bells: http://lyricstrue.net/bandsongtext/Unknown/Farnesol.html.
Self-healing polymers. Biological materials incur damage or decay over time; biological processes restore them. But ordinary chemical materials? Now there is work to design a polymeric material with self-repair capability. http://autonomic.beckman.illinois.edu/. (The work described at this site was pioneering work on self-healing polymers. More has followed. One interesting new approach is described in a 2008 paper now listed in the Ch 14 handout.)
Recycling information: https://www.contracosta.ca.gov/4911/Recycling. Among other things, check the "Reuse and Recycling Guide." From Contra Costa County; includes links to recycling agencies in some other local areas. [An article about the merits of burning vs burying plastics, Piasecki et al (1998), is on the Further reading: Old articles web page.]
Alameda County recycling: https://www.stopwaste.org/
A kit that lets you make your own C60 "buckyballs" is available, on the Download page. The kit may be suitable for ages 8+.
The The E-Z system for naming alkenes; examples of using the CIP rules. Ouellette covers this fine in Ch 4. The page now includes more examples, including how to apply the CIP priority rules.
Some posts in my Musings newsletter on the topics of this section have been noted above. Others include...
* A new form of carbon: C18 (September 24, 2019).
My Musings newsletter includes posts on aromatic compounds. Many of these are listed below, in two lists. List 1 focuses on graphene and carbon nanotubes. List 2 is general.
List 1. Posts in my Musings newsletter on graphene, carbon nanotubes, and such include:
* Goldene: 2-dimensional gold (July 9, 2024). Not carbon at all, but ...
* Graphene-based sensors for capturing your brain waves (April 22, 2023).
* Graphullerene (March 20, 2023).
* Ultra-fast PCR (March 14, 2023).
* Briefly noted... Making carbon nanotubes out of waste face masks (December 7, 2022).
* Graphdiyne: helping make silver a better anti-bacterial agent (June 11, 2022).
* A better membrane for vanadium-based flow batteries (March 11, 2022).
* A new form of carbon -- hard enough to scratch diamond (March 1, 2022).
* Not graphene: a new flat form of carbon sheets (June 12, 2021).
* Electronic monitoring of plant health; it might even allow an injured plant to call a doctor (June 21, 2020).
* What if CRISPR teamed up with graphene? (September 6, 2019).
* A thermal management system, based on carbon nanotubes, for your jacket (May 3, 2019).
* GO dough (April 9, 2019).
* Making carbon nanotubes from captured carbon dioxide (June 3, 2018).
* Coloring with graphene: making a warning system for structural cracks? (June 2, 2017).
* Water desalination using graphene oxide membranes? (April 29, 2017).
* A better way to get to Alpha Centauri? (March 15, 2017).
* How do you get silkworms to make stronger silk, reinforced with graphene? (October 24, 2016).
* Supercapacitors in the form of stretchable fibers -- suitable for clothing (May 2, 2014).
* A simple way to make a supercapacitor with high energy storage? (January 6, 2014).
* Characterization of carbon nanotubes (December 3, 2013).
* Loudspeakers: From gold-coated pig intestine to graphene (April 27, 2013).
* Graphene bubbles: tiny adjustable lenses? (January 15, 2012).
* A box that will fold up upon command -- heat- or light-actuated switches (September 3, 2011).
* Image of a carbon atom that isn't there (August 17, 2008).List 2. Other relevant Musings posts include:
* Briefly noted... A Möbius strip made of carbon atoms (September 7, 2022).
* Understanding locust swarming: a chemical to deal with the problem? (October 13, 2020).
* Hückel at 40 -- that's n = 40: the largest known aromatic ring (February 1, 2020).
* Fish may adapt to pollution by stealing genes from another species (July 21, 2019).
* Progress toward making a homogeneous product from diverse lignins (May 17, 2019).
* How bacteria make toluene (May 18, 2018).
* Does using printer toner lead to carcinogens? (October 31, 2017).
* The longest acene (September 6, 2017).
* Making triangulene -- one molecule at a time (March 29, 2017).
* How many atoms can one carbon atom bond to? (January 14, 2017).
* A simpler way to make styrene (July 10, 2015).
Turning trash into graphene. It's the miracle material of our time -- but too expensive to actually use. A recent article shows that graphene can be made inexpensively by heating most any carbon-containing material, including waste food and plastic, to 3000 K. The flash heating is done within a fraction of a second by the discharge of a capacitor. It's hot enough to break most of the chemical bonds in the material. Most of the non-C atoms are released as gases. Upon rapid cooling, most of the C forms high-quality graphene. The work in the article is at gram scale, but the authors think the method can be scaled up to tons/day -- and they have formed a company to do so. An interesting development; we'll see how it turns out.
* News stories...
- New method turns any carbon waste into graphene. (scientiststudy.com, February 2, 2020. Now archived.) Gives full article information, but without a live link.
- Behind the paper: Gram-scale bottom-up flash graphene synthesis -- A magic wand has been developed! A touch, a bolt of electricity, and a bright flash of light turns trash into the greatest of space-age materials: graphene. (J M Tour, Nature Research Device and Materials Engineering Community, February 3, 2020.) By the senior author.
* Direct link to the article: Gram-scale bottom-up flash graphene synthesis. (D X Luong et al, Nature 577:647, January 30, 2020.)
A new 1980s-quality computer. It doesn't use silicon transistors. It is based entirely on CNT (carbon nanotube) transistors. The current article represents major progress in making a functional computer based on CNTs. The future? We'll just have to wait, but some think CNTs could be the way to go.
* News story: A Carbon Nanotube Microprocessor Mature Enough to Say Hello -- Three new breakthroughs make commercial nanotube processors possible. (S K Moore, IEEE Spectrum, August 28, 2019.) Links to the article. (The three brekthroughs are RINSE, DREAM, and MIXED.)
Using graphene as a hair dye. It has merit, and might even be practical.
* News story: Scientists Have Found a Way to Use Graphene As... Hair Dye. (J Bowler, Science Alert, March 17, 2018.)
* The article: Multifunctional Graphene Hair Dye. (C Luo et al, Chem 4:784, April 12, 2018.)
* I learned of this at a "nano" seminar by senior author Jiaxing Huang, Professor of Materials Science at Northwestern University. He thinks it is good to explore unusual ideas! Check out his "Teaching stories" page.
For more about graphene from the same lab, see the Musings post: GO dough (April 9, 2019).
Dr Tom Newton at the University of Southern Maine developed an animation showing the bonding in benzene in terms of orbitals. Here is a copy of the figure: Newton's Figure 2, on the aromatic ring [link opens in new window]. In particular, note the loop formed by six p orbitals, one from each bonding C, overlapping sideways. This continuous loop of sideways-overlapping p orbitals, with six electrons, is responsible for the aromatic character. This item builds on the item posted above for Alkenes on π bonding in alkenes, also from Dr Newton. (This page also gives you more explanation of the basis of Hückel's rule, though the level is fairly difficult.) For a broader view of what aromaticity entails, see my page on this topic, listed in this section below. (Newton's organic chemistry web site is no longer available.)
The 2010 Physics Nobel Prize was awarded to Andre Geim and Konstantin Novoselov, University of Manchester, "for groundbreaking experiments regarding the two-dimensional material graphene". See the Nobel page The Nobel Prize in Physics 2010.
Book. Listed on my page of Books: Suggestions for general science reading... Johnson & Meany, Graphene -- The superstrong, superthin and superversatile material that will revolutionize the world (2018).
The following pages at my site are relevant to this chapter:
The Phenyl group page will help you with this and related terms. The page shows structures.
The Aromatic page addresses the broader question, What does "aromatic" really mean? I encourage you to browse it, and enjoy the pictures!
Dr Phil Bays (St. Mary's College, Indiana) has a good set of practice problems on stereochemistry. The structures are clear, and the questions appropriate. Instant feedback. http://sites.saintmarys.edu/~pbays/Stereochemistry.html. (Part of this site was recommended, above, for use with cycloalkanes.)
The 2001 Nobel prize in Chemistry was awarded to three scientists for their work in developing practical methods for making the correct isomer of drugs, that is for doing chiral synthesis. See the Nobel site, https://www.nobelprize.org/prizes/chemistry/2001/summary/. Also see "Further reading" items on this topic, in chapter handout.
Everything has a mirror image? Even Google? Try it, at https://elgoog.im/. (The story is that this was introduced as an attempt to circumvent censorship of search engines in certain countries. It worked.) This site is also featured in the Musings post elgooG (October 12, 2009).
Ch 6 introduces the R,S system for naming chiral isomers. As noted, The E,Z system is a rigorous IUPAC system for naming alkene isomers, using the same general approach -- and same priority rules. See my page The E-Z system for naming alkenes; examples of using the CIP rules. (This was mentioned earlier, under Alkenes.)
You may also want to look at some stereoisomers of chiral compounds in RasMol. See my RasMol page.
H Prior et al, Mirror-induced behavior in the magpie (Pica pica): Evidence of self-recognition. PLoS Biol 6(8):e202, 8/08. The ability to recognize oneself in a mirror is a trait sometimes considered characteristic of only humans and apes. Over recent years, evidence accumulated that elephants and dolphins, too, show this aspect of self-recognition. Here is evidence that some birds recognize themselves in the mirror. Very readable paper, with interesting methodology. The paper is freely available online: https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.0060202. The article is listed in the handout for Ch 6. It is also the subject of a post in my Musings newsletter: Self (October 8, 2008).
Other related posts in my Musings newsletter include...
* Briefly noted... A Möbius strip made of carbon atoms (September 7, 2022).
* CISS: separating mirror-image molecules using a magnetic field? (August 7, 2018).
For animations of elimination and substitution reactions, see https://homepages.bluffton.edu/~bergerd/classes/CEM221/sn-e/home.html. These are types of reactions used in Sections 8.5 & 8.6; they were introduced, with mechanisms, in Ch 7, which we skip. These animations will usefully reinforce these basic reaction types even if you do not worry about the mechanisms for now. For those going on in organic chemistry, this is essential material -- and these are nice animations. (Both Flash and gif animations are available. If possible, try the Flash animations first.) From Daniel Berger, Bluffton College.
Removing stains at home, from the Department of Fiber Science & Apparel Design at Cornell. A practical guide to removing stains. Chemistry underlies much of this... what is soluble in what, largely determined by polarity. Go to the Department's outreach page: https://www.human.cornell.edu/fsad/engagement. Scroll down to "Stain removal for the home" (pdf file).
MTBE = methyl t-butyl ether; it is a controversial gasoline additive. A report from the National Academy of Sciences summarizes the key issues in the debate on oxygenated fuels (using mainly MTBE or ethanol). Go to the National Academy Press, at https://www.nationalacademies.org/. Search on MTBE. Doing this will give you any current information that may be available there, but the main point for now is the 1996 book "Toxicological and performance aspects of oxygenated motor fuels." I suggest you start with the Executive Summary.
Thimerosal. This is the mercury-containing compound that is used as a preservative in some vaccines. It is controversial because of concerns about possible toxicity. It is relevant here because the Hg is attached to a sulfur atom; one can think of it as a Hg-substituted thiol. I have posted two items on this. My thimerosal page shows the structure of thimerosal and some related compounds, including aspirin. I have also posted a short note about thimerosal on BITN - Miscellaneous; section on vaccines. This includes a link to an FDA site, which should be a good starting point for a broader consideration of the compound. The FDA page should not necessarily be considered a final answer, but it is well organized, and should serve to at least outline the issues. Let me know of questions. This topic is also listed under Introductory Chemistry Internet Resources: Thimerosal.
A particular question that came up was about the behavior of methyl mercury vs ethyl mercury. My bottom line is that this is not very clear. Although there is some evidence that the ethyl mercury may be less toxic, the evidence seems very limited. There are two factors to consider in making this comparison. One is chemical/physical behavior. Both are non-polar, and thus relatively soluble in fat. Offhand, I would not want to predict any difference between them on this point. The second issue is their precise biological action. I don't know of any detailed work on this; as already noted, the evidence that the ethyl mercury may be less toxic is weak, and I have not seen any detailed analysis or explanation of it.
Also see the section on Energy resources. Some of the items it contains used to be listed in this section or under Introduction; alkanes.
The following posts in my Musings newsletter are about aldehydes and ketones:
* What's the connection: blue cheese, rotten coconuts, and the odorous house ant? (August 24, 2015).
* Alcohol consumption, an "ethnic" mutation, and a possible new drug (October 28, 2014).
"I Have Seen the Light! Vision and Light-Induced Molecular Changes." How vision works, including the chemistry of the photoreceptor. That chemistry involves an aldehyde reaction, and also cis-trans isomerization. https://web.archive.org/web/20200121175448/http://www.chemistry.wustl.edu/~edudev/LabTutorials/ (now archived). Includes good graphics, at molecular and biological levels. From R Casiday and R Frey of Washington University St Louis. (Another of their set, on oxidative phosphorylation, is listed below, for Metabolism.)
Salivary amylase is the first enzyme that helps us digest starch -- in the mouth. Humans in societies with high starch consumption have more salivary amylase -- and more copies of the gene for it -- than humans in societies with low starch consumption. This suggests that natural selection has been occurring in humans for this trait, and also supports the emerging idea that copy number variation is important. The paper also notes limited data supporting the same correlation with other primates. This work was published as G H Perry et al, Diet and the evolution of human amylase gene copy number variation. Nature Genetics 39:1256, 10/07. It is listed in the chapter handout under Novembre et al, the accompanying news story in the same issue. A news story that is freely available: Starch 'fuel of human evolution' -- Man's ability to digest starchy foods like the potato may explain our success on the planet, genetic work suggests. http://news.bbc.co.uk/1/hi/health/6983330.stm.
An interesting story of the development of an artificial sweetener, using an uncommon and unmetabolized sugar. The article also discusses the sweetness of L-sugars; these might be suitable as artificial sweeteners, but are not economically practical. "A natural way to stay sweet": https://spinoff.nasa.gov/Spinoff2004/ch_4.html.
|
Electricigenic bacteria -- bacteria that can couple their electron transport directly to an external electrode, and thus can serve as the basis of a microbial fuel cell. One possible application is the use of waste organics, including sugars, to make electricity. One of the examples most studied is the genus Geobacter (a genus that does not itself use sugar).
* This is Fig 4 of: D Lovley, Microbial energizers: Fuel cells that keep on going. Microbe 1:323, 7/06. It is freely available at: https://www.asmscience.org/content/journal/microbe/10.1128/microbe.1.323.1. |

|
Glycomics. Article: A A Weiss & S S Iyer, Glycomics aims to interpret the third molecular language of cells. Microbe 2:489, 10/07. Free online: https://www.asmscience.org/content/journal/microbe/2/10. Scroll down to the item. A nice overview, written for a general audience, of the role of carbohydrates on cell surfaces. The sugars are found as part of glycolipids and glycoproteins (more about these in later chapters, on lipids and proteins). Many receptors for viruses and toxins involve sugar residues -- hence the connection to microbiology. The article discusses influenza virus and botulinum toxin among the examples where the sugar residues are key in determining the specificity of the agent.
Here is a (partial) list of posts in my Musings newsletter that are relevant to this topic. (Many posts on polysaccharides are not listed here.)
* Added September 17, 2025. Sugar in drinks vs sugar in solid foods: Do they have different effects? (September 17, 2025).
* An edible food wrapper, based on bacterial cellulose (February 28, 2024).
* Acholetin: a new polymer from bacteria (June 4, 2022).
* A new role for RNA (August 3, 2021).
* Low-carb diets: Long-term effects? (September 4, 2018).
* The strongest bio-material? (May 30, 2018).
* Influence of the food additive trehalose on Clostridium difficile? (February 23, 2018).
* History of plastic -- by the numbers (October 23, 2017).
* Some shrimp in your wine? (August 27, 2016).
* Breastfeeding and obesity: the HMO and microbiome connections? (November 14, 2015).
* A better way to divide 6 by 2: A more efficient way to use sugar (November 10, 2013).
* Why and how some cockroaches avoid glucose (October 11, 2013).
* Fructose and your brain (January 28, 2013).
* Fructose; soft drinks vs fruit juices (November 7, 2010).
For more about polymers, see the Macrogalleria site, listed above for Alkenes.
Here are some posts in my Musings newsletter relevant here. Most are on plastics.
* Good enzymatic degradation of polyesters, by manufacturing the plastic with the enzymes in it (May 4, 2021).
* Follow-up: bacterial degradation of PET plastic (April 25, 2018).
* A "greener" way to make acrylonitrile? (January 6, 2018).
* Better chocolate? Use better yeast? (May 3, 2016). Esters and flavors.
* Discovery of bacteria that degrade PET plastic (April 3, 2016).
* The advantage of washing with formic acid (August 8, 2014).
Here is a list of some posts in my Musings newsletter that are relevant to this topic. The list includes posts on fat metabolism and obesity.
* Old baby bottles (November 11, 2019).
* Cats, fats, and Toxoplasma. And mice. (October 21, 2019).
* Omega-3 fatty acids and the adaptation of fish to fresh water (August 3, 2019).
* A scientific article about Christmas in the journal Atherosclerosis (February 3, 2019).
* Using a zipper to prevent obesity (September 25, 2018).
* Treating obesity: A microneedle patch to induce local fat browning (January 5, 2018).
* Improving soybean oil by using high voltage plasma (January 9, 2017).
* Improving soybean oil by gene editing (January 8, 2017).
* Bee history (February 13, 2016).
* Breastfeeding and obesity: the HMO and microbiome connections? (November 14, 2015).
* An obesity gene: control of brown fat (October 2, 2015).
* How flippase works (September 25, 2015).
* What's the connection: blue cheese, rotten coconuts, and the odorous house ant? (August 24, 2015).
* Measuring the level of a non-existent hormone (April 10, 2015). Links to an update.
* Mutations that lead to reduced risk for heart disease (November 21, 2014).
* Cachexia: is it BAT run amok? (September 22, 2014).
* Could we treat obesity with probiotic bacteria? (August 5, 2014).
* YY in the mouth? (April 4, 2014).
* Why exercise is good for you, BAIBA (March 10, 2014).
* Brown fat: different kinds respond differently to cold (September 20, 2013).
* Antibiotics and obesity: Is there a causal connection? (October 15, 2012).
* How good is "good cholesterol" (HDL)? (September 21, 2012).
* Bacteria induce simple "pre-animal" to become colonial (September 8, 2012).
* Heart health and python blood (December 28, 2011).
* How to find the blood (August 29, 2011).
* A gene for obesity (May 7, 2011).
* A virus associated with obesity? (October 4, 2010).
* Turning sewage into profit -- via rocket fuel (September 15, 2010).
* Obesity, gut bacteria, and the immune system (May 24, 2010).
* Omega-3 fatty acids; fish oil (March 29, 2010).
* How do you make phospholipid membranes if you are short of phosphorus? (November 1, 2009).
* Brown fat (May 21, 2009).
A new type of hormone. An idea that has been developing in recent years is that adipose (fat) tissue is "active" -- that it sends out hormonal signals that affect metabolism. The first and most famous of the adipokines -- hormones made by adipose tissue -- is leptin. Now we have evidence, from mice, for an adipokine that is itself a fatty acid. The fatty acid is cis-9-hexadecenoic acid, popularly called palmitoleic acid. A news story: A New Class of Hormone from Healthy Fat Cells Benefits Body Metabolism, HSPH Researchers Find in Mice -- Discovery of 'lipokine' signaling could eventually lead to new treatments for obesity-related conditions. September 18, 2008. https://www.hsph.harvard.edu/news/press-releases/new-class-of-hormone-from-healthy-fat-cells-benefits-body-metabolism-mice/. The article, which is listed in the chapter handout, is: H Cao et al, Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134:933-944, 9/19/08.
Stress and neuropeptide Y. Stress may lead to fat accumulation. In mice, it has now been shown that part of this story is the key role of neuropeptide Y (NPY). News story: Scientists Discover How Stress Causes Obesity And How Fat Can Be Removed Using A Simple Injection. https://www.medicalnewstoday.com/articles/75768. The paper is L E Kuo et al, Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nature Medicine 13(7):803, 7/07. Accompanying news story: J P Warne & M F Dallman, Stress, diet and abdominal obesity: Y? Nature Medicine 13(7):781, 7/07.
Leptin in baby formula? In mice, giving large doses of the hormone leptin during infancy ensures that they never become obese -- and never get diabetes. Would this work in humans? It is being discussed. A news story: Scientists working on formula milk that prevents child obesity. https://www.theguardian.com/science/2007/apr/23/medicalresearch.ethicsofscience. (The Guardian, April 22, 2007.)
Aspirin works by inhibiting cyclooxygenase (COX), a key enzyme in the synthesis of prostaglandins from arachidonic acid (p 372). More recently, we have understood that there are (at least) two COX enzymes, with distinct biological roles. This knowledge led to the development of a new class of anti-inflammatory drugs that specifically inhibit COX-2 (e.g., Celebrex and Vioxx), thus avoiding some of the classic side effects of aspirin. The recent news has been about problems with these drugs -- and perhaps with a wider range of anti-inflammatory drugs. The following site, from the FDA, provides information on these drug issues: COX-2 Selective (includes Bextra, Celebrex, and Vioxx) and Non-Selective Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), https://web.archive.org/web/20190207143853/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM103420 (now archived). Among other things, see the "Questions and Answers".
The news referred to above certainly has a negative tone, as the emphasis has been on negative effects of the drugs, and perhaps the failure of our drug regulation system to recognize these effects soon enough. But also bear in mind that there is a fascinating biology story developing here. We start with a common useful drug (aspirin), long used with no understanding of how it works. We now have an idea how it works, and that story gets more and more complicated as we learn more. The current drug story has at least two underlying causes: the drugs are not as specific as we sometimes say in casual discussion, and the roles of the COX enzymes are more complex than we suspected. More will be learned, for better and worse. There are at least hints that COX inhibitors may be useful for treating some cancer and other diseases.
FDA page on Trans Fat Now Listed With Saturated Fat and Cholesterol. A page of consumer-oriented information about trans fatty acids (TFA), and about food labeling. https://web.archive.org/web/20171114122150/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm274590.htm. (Now archived.) Also in Spanish.
The zero trans fat cooking oil contest and related materials. The goal is to help restaurants eliminate the use of trans-fats in cooking. Useful information, and fun. http://frytest.com/index.php.
The following sites have good sections on biological membranes, including the role of cholesterol.
* http://cytochemistry.net/cell-biology/membrane_intro.htm. From Gwen Childs, University of Arkansas for Medical Sciences.
* https://en.wikibooks.org/wiki/Cell_Biology. Scroll down to: Parts of the cell, Membranes. From Wikibooks, the open-content textbooks collection; this book is based on the cell biology textbook started by Mark Dalton, University of Minnesota.
The 2003 Nobel prize in chemistry was awarded to two scientists for their work on transport channels, for water and ions, in biological membranes. For more: https://www.nobelprize.org/prizes/chemistry/2003/summary/
The 1982 Nobel prize in medicine was awarded to three scientists "for their discoveries concerning prostaglandins and related biologically active substances." For more: https://www.nobelprize.org/prizes/medicine/1982/summary/.
e.hormone: Environmental estrogens and other hormones. The general theme is hormone mimics: chemicals that are not a normal part of the human body but which may behave like hormones in the body. These include chemicals found in nature either naturally (e.g., the so-called phytoestrogens in plants) or derived from pesticides etc. The site is from the Center for Bioenvironmental Research, at Tulane and Xavier Universities, in New Orleans. http://e.hormone.tulane.edu/
For more references related to the broad topic of Lipids, especially those with medical implications, see my page Further reading: medical topics.
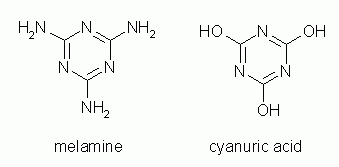 Spring 2007 brought stories of pets dying from contaminated pet food. Why? Various theories were brought forward, some with little credibility. In fact, the one that now seems to be correct had little credibility at first. The melamine story. Melamine is an organic amine used to make plastics known as, well, melamines. For example, a plastic made with melamine and formaldehyde is Formica. Apparently it may have been added to commercial wheat gluten in order to enhance the apparent protein content. After all, melamine has lots of N, and the common protein assay simply measures N. However gruesome this might seem, the story had a flaw, a fairly serious flaw. Melamine is a well known chemical, and it just isn't very toxic. Well, it seems that melamine was indeed the culprit, but not alone. The problem was a combination of two compounds, melamine and cyanuric acid. From looking at their structures, one can easily see how they could interact with each other. Together, they are much less soluble than either alone. And indeed the crystals found in the urinary tracts of the poisoned animals contained melamine + cyanuric acid. (Where the cyanuric acid came from was originally unclear, but it now seems that it was in the gluten, too.)
Spring 2007 brought stories of pets dying from contaminated pet food. Why? Various theories were brought forward, some with little credibility. In fact, the one that now seems to be correct had little credibility at first. The melamine story. Melamine is an organic amine used to make plastics known as, well, melamines. For example, a plastic made with melamine and formaldehyde is Formica. Apparently it may have been added to commercial wheat gluten in order to enhance the apparent protein content. After all, melamine has lots of N, and the common protein assay simply measures N. However gruesome this might seem, the story had a flaw, a fairly serious flaw. Melamine is a well known chemical, and it just isn't very toxic. Well, it seems that melamine was indeed the culprit, but not alone. The problem was a combination of two compounds, melamine and cyanuric acid. From looking at their structures, one can easily see how they could interact with each other. Together, they are much less soluble than either alone. And indeed the crystals found in the urinary tracts of the poisoned animals contained melamine + cyanuric acid. (Where the cyanuric acid came from was originally unclear, but it now seems that it was in the gluten, too.)
The following two links give glimpses of this story as it emerged.
* How two innocuous compounds combined to kill pets. A news story, May 7, 2007. https://www.washingtonpost.com/wp-dyn/content/article/2007/05/06/AR2007050601034.html.
* Melamine Pet Food Recall - Frequently Asked Questions. A site from the US FDA Center for Veterinary Medicine that was actively maintained during the pet food incident. https://web.archive.org/web/20220209233153/https://www.fda.gov/animal-veterinary/recalls-withdrawals/melamine-pet-food-recall-frequently-asked-questions. Now archived.Fall 2008, and more melamine. In food for humans. Details not clear at this writing, but it is the same melamine discussed above. Apparently, it was added to milk, to boost the apparent protein content of the milk. The milk, in turn, gets used for a range of products, including infant formula.
Two sites on the melamine contamination of milk products:
* Melamine Contamination in China. (1/9/09.) An FDA page, now archived at: https://web.archive.org/web/20210226132659/https://foodconsumer.org/7777/8888/L_aws_amp_P_olitics_42/010908392009_Melamine_Contamination_in_China.shtml.
* Wikipedia: 2008 Chinese milk scandal. https://en.wikipedia.org/wiki/2008_Chinese_milk_scandal.An article analyzing the pet food incident has been published. The paper describes the incident, the identification of melamine and cyanuric acid -- both in the gluten, and the toxicology of individual components as well as mixtures. Most of the work is from Procter & Gamble. The paper is: R L M Dobson et al, Identification and Characterization of Toxicity of Contaminants in Pet Food Leading to an Outbreak of Renal Toxicity in Cats and Dogs. Toxicological Sciences, 106(1):251-262, 11/08. The abstract -- very informative -- is available at PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18689873.
Posts in my Musings newsletter that are relevant to this topic include:
* The possible role of bacteria in determining the toxicity of melamine... Melamine toxicity: possible role of gut microbiota (April 21, 2013).
* A study of the possible origin of RNA, turns out to deal with chemical subunits very similar to those discussed here... A novel type of polymer -- and its possible relevance to the origin of life (March 15, 2013).
For more about the properties of amides, see my page on Amides. The page discusses the non-basicity and the planarity of the amide linkage, and presents the resonance structures that account for these properties. Also relevant to Ouellette Ch 15, on proteins.
The 2002 Nobel Prize in Chemistry was awarded to three scientists for the development of methods for use of mass spectrometry (MS) and nuclear magnetic resonance (NMR) with proteins. See the Nobel site: https://www.nobelprize.org/prizes/chemistry/2002/summary/. Also see related articles in JCE: 1) M S Vestling, Using mass spectrometry for proteins. 2) S Cavagnero, Using NMR to determine protein structure in solution. J Chem Educ 80:122 & 125, 2/03.
The 2004 Nobel Prize in Chemistry was awarded to three scientists for their key roles in understanding how proteins are degraded. Specifically, they discovered the role of ubiquitin, a special protein that is attached to proteins to tag them for degradation. The bigger story is the increasing recognition of the importance of protein degradation. Many proteins are made defective, and must be degraded rapidly. Some proteins are supposed to act for only a brief time, and must be promptly degraded. And some mutations lead to proteins that are unstable, and get marked for rapid degradation. See the Nobel site: https://www.nobelprize.org/prizes/chemistry/2004/summary/. This site is also listed for Internet resources for Molecular Biology: Transcription - eukaryotes.
For more about the properties of peptide bonds, which are amide linkages, see my page on Amides. The page discusses the non-basicity and the planarity of the amide linkage, and presents the resonance structures that account for these properties. This page was originally mentioned along with Ch 14, on amides.
J Winkler, Misfolded proteins and Parkinson's disease. Engineering & Science Vol LXVIII #3, 2005, p 14. Available online: http://calteches.library.caltech.edu/4140/. This article is based on a public lecture, and much of it should be at a level appropriate for X402 students. It generally discusses proteins and how they fold, and focuses on the specific case of a protein whose misfolding is implicated in Parkinson's disease.
C Wong Po Foo et al, Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. PNAS 103:9428-9433, 6/20/06. Silk protein is a classic example of a fibrous protein rich in (-sheets (p 439). It is a particularly interesting protein because of its strength. Silk strength depends both on the source and on the spinning technique. Silkworm silk and spider silk both get much attention, and one company is producing spider silk in goat milk. In this paper, they explore making composite materials, containing silk and silica -- somewhat like what is found in diatom skeletons. They start by making a chimeric protein that contains silk plus key regions of diatom proteins required to promote bio-silica formation. Available online: https://www.pnas.org/content/103/25/9428.
There are posts in my Musings newsletter about developments with silk, both from spider and silkworm. These include:
* Medical safety: What if pills were better labeled, so your phone could verify them? (April 23, 2022).
* A rigid silk basket (November 10, 2020).
* Stabilizing broken bones: could we use spider silk instead of metal plates? (June 24, 2018).
* How do you get silkworms to make stronger silk, reinforced with graphene? (October 24, 2016).
* Silk-clothed electronic devices that disappear when you are done with them (October 19, 2012).
* Silk: Stabilizing vaccines and drugs (July 29, 2012).
* Spiders and violins (May 4, 2012).
* Spider silk: Can you teach an old silkworm new tricks? -- Update (February 11, 2012).Musings posts about making proteins (peptides) include...
* The role of tiny droplets in driving unfavorable reactions -- such as those needed to get life started (January 17, 2023).
* Encoding peptides from primordial RNA? (June 6, 2022).
* Making peptides in space: a new pathway (April 15, 2022).Musings posts about amino acids include...
* Modifying bacteria, with help from a bird, so they can make and use an unusual amino acid (October 8, 2022).
* Using bacteria to treat phenylketonuria? (September 16, 2018).
* A better way to make (the dye for) blue jeans, using bacteria? (March 5, 2018).
* NASA finds a chemical of life on a comet (September 1, 2009).
A Protein Primer: A Musical Introduction to Protein Structure. Assign pitches to the amino acids -- etc etc etc. Certainly fun, and possibly even a useful introduction to protein structure. Covers the genetic code and various levels of protein structure. The site has several MP3 files to illustrate the system. https://www.whozoo.org/mac/Music/Primer/Primer_index.htm. From M A Clark, Texas Wesleyan University.
For more related to this chapter, see my page of Molecular Biology Internet resources, especially the section on Protein synthesis. The level of that page is generally more advanced than this page, but there is considerable overlap.
The web site for Worthington Biochemical Corp contains a nice Introduction to Enzymes, which they maintain for the benefit of beginning biology or biochem students. https://www.worthington-biochem.com/tools-resources/intro-to-enzymes. This is a required reading assignment for my X402 class. A pdf version is available there, or you can click on topics.
The small metabolic chart I show is available online from Sigma, as a pdf file. Go to Sigma's Metabolic Pathways page, part of their Enzyme Explorer, https://www.sigmaaldrich.com/life-science/metabolomics/learning-center/metabolic-pathways.html. This page gives you the link to the pdf file; if you would like a paper copy, see the purchase information on the page.
That Metabolic Pathways page at Sigma, above, also includes additional resources on metabolism. There are several animations of metabolic processes, and a link to a list of enzymes by EC (Enzyme Commission) number.
A famous larger chart, called "Biochemical Pathways", was long distributed by Boehringer Mannheim and later by Roche. They have decided to drop this project, but a copy of the chart is available at Internet Archive.
Many journal articles are available online, though free access may be restricted. I list here only occasional ones, that I know are open to free access. Others may have free access, and many more will be available to those who use the university access.
F Berg et al, The Uncoupling Protein 1 gene (UCP1) is disrupted in the pig lineage: A genetic explanation for poor thermoregulation in piglets. PLoS Genetics 2(8):e129, 8/06. If we could just burn our food without capturing the energy, we could eat more with less weight gain. A way to avoid energy capture would be to somehow dissipate the energy from NADH oxidation, avoiding coupling it to ATP formation. In fact, "uncoupling proteins" (UCP) allow just that. Mice have them, in brown fat. Humans have them, but only in infancy. Pigs do not have them, and this paper explores the genetic basis of that defect. Free online at: https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.0020129.
Physiology of Respiration. http://historyofscience.free.fr/Lavoisier-Friends/a_chap4_lavoisier.html. This page describes how Lavoisier made the connection between ordinary combustion and burning our food. From Jean Pierre Poirier author of "Lavoisier, Chemist, Biologist, Economist" (University of Pennsylvania Press, 1996). I have also listed this page under Internet Resources for Intro Chemistry: Reactions, and I have listed the larger Lavoisier site under Miscellaneous Chemistry Internet Resources: History.
A good source of the complete glycolysis pathway is https://www.bio.davidson.edu/Courses/Molbio/MolStudents/spring2005/Gemberling/protein.html. From a student at Davidson College. The page is on a specific enzyme, but includes the complete glycolysis pathway. You can use this to check yourself if you do Part 1 of the Glycolysis worksheet (my supplementary handout; see Metabolism), or you can just use it as a source of the pathway.
An extensive tutorial on oxidative phosphorylation is included in a fine collection of tutorials at Washington Univ (St Louis) that generally aim to show practical aspects of freshman chemistry. Go to their main site (now archived), https://web.archive.org/web/20200129094207/http://www.chemistry.wustl.edu/~edudev/LabTutorials/, and choose "Energy for the Body: Oxidative phosphorylation". This tutorial includes a quite complex animation of the electron transfer process. Of course, while at the Tutorials site, look around for others that may interest you. (Another of this set, on the biochemistry of vision, is listed above, for Aldehydes and ketones.)
Electron transport and ATP formation. Dr Thomas Terry, Univ of Connecticut, has made available a couple of pages on these processes, including some nice animations. These are now archived: http://web.archive.org/web/20110720074531/http://www.sp.uconn.edu/~terry/images/anim/ETS.html. That page is "Animation of Electron Transport in Mitochondria", and it links to a second page, "Animation of ATP synthesis in Mitochondria".
The final enzyme in the oxidative phosphorylation sequence is the ATPase. This enzyme actually rotates in the membrane as it works to make ATP. The following site shows diagrams of this complex enzyme. It also has various animations. It is part of a more extensive site on metabolism.
* http://www.k2.phys.waseda.ac.jp/Movies.html. There are several movies listed for "Rotation of F1-ATPase" -- and other topics. From the Kinosita lab, on Single Molecule Physiology, Waseda University.
Recall the Metabolic Pathways page at Sigma, listed above under Metabolic Charts. It also includes additional resources on metabolism, with animations of metabolic processes, including the ATPase.
For fun... some songs about metabolism:
* Kevin Ahern's Wildly Popular Metabolic Melodies, original songs from Kevin Ahern, Oregon State Univ: archive.
* The Biochemists' Songbook, by Harold Baum. MP3 files of recordings originally released with Dr Baum's book, and posted by biochem prof Jeff Cohlberg, with the author's permission. https://sites.google.com/view/biochemists-songbook. Links to more.
Although we do not discuss spectroscopy in X402, it is an important topic in university level organic chemistry. Ouellette's Chapter 18 is a good introduction, and problems are scattered throughout the book (typically at the end of the set of problems for each functional group). Here are some web sites with good presentations of aspects of spectroscopy. Some of these have good animations, and good practice problems.
Thanks to M. Farooq Wahab (Chemistry, University of Karachi) for help getting this section started, and for contributing many of the listings based on his teaching experiences.
https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/spectro.htm. Introduction to Spectroscopy. Includes mass spectrometry (MS), ultraviolet-visible spectroscopy (UV-Vis), infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR), plus links to other sources of information and practice problems. From W Reusch (Michigan State Univ), as part of his online textbook of organic chemistry, which is listed below under General.
http://www.nmrdb.org/predictor/. NMR simulation. Draw a molecule, and the web page will draw the expected NMR spectrum. To use it: Find the section labeled "How to proceed ?", at the right. Choose "1. Draw a molecule". Play a little and you will figure out the drawing program. When you have drawn the molecule, press the "Submit molecule" button at the bottom of the Draw window. Note that other options are available for providing the molecule information. (From Luc Patiny, Ecole Polytechnique Federale de Lausanne.)
http://www.ionsource.com. A commercial site, with a major section of educational information, Mass Spectrometry and Biotechnology Resource, by Andrew Guzzetta. Covers mass spectrometry, HPLC, and more.
Why is water blue? https://web.archive.org/web/20200209091327/http://www.dartmouth.edu/~etrnsfer/water.htm. (Now archived.) Discusses in both general and technical terms how the weak blue color of water is related to its structure, including the hydrogen bonding in liquid water. The evidence is based largely on analysis of the wavelengths of vibrational transitions, seen in visible and infra-red spectra. Interestingly, they predict and show that water with the heavy isotope of H, 2H (deuterium), is much less blue. From Charles Braun and Sergei Smirnov at Dartmouth. A delightful page, based on an article originally published in J Chem Educ (70:612, August 1993), but now enhanced with color figures. This page is also listed on my page of Internet resources for Introductory chemistry under Water.
Also see above section on Amino acids, proteins, genes, for an item on the 2002 Nobel Prize in Chemistry for the use of NMR and MS with proteins.
Also see Medicine: Color vision and color blindness section of my Internet resources: Biology - Miscellaneous page for information on the nature of light and color vision.
This is a list of posts in my Musings newsletter on energy. It includes energy issues at a range of levels, from how society uses energy to the biochemical pathways of energy production in particular organisms. It also includes some posts on the related issues of greenhouse gases and climate change.
Some of the posts listed here are not really about organic or biochemistry. They are included as some examples of energy work.
* Added September 23, 2025.
Using onions to improve solar cells (September 23, 2025).
* A nuclear-powered battery for your phone? (October 9, 2024).
* Storing hydrogen in magnesium diboride nanosheets (May 15, 2023).
* Using abandoned mines to store energy (January 30, 2023).
* Storing hydrogen in salts (such as potassium formate) (November 7, 2022).
* If your computer was powered by photosynthesis, would you have to water it? (August 29, 2022).
* A tower of (solar) power -- which makes kerosene (August 22, 2022).
* A battery made of paper, and activated by a drop of water (August 6, 2022).
* Growing food with artificial photosynthesis? (July 9, 2022).
* What to do with silicon waste from dead solar cells (May 23, 2022).
* A solar cell that generates electricity at night (April 12, 2022).
* A better membrane for vanadium-based flow batteries (March 11, 2022).
* Briefly noted... Electronic structure of lithium atoms (March 8, 2022).
* Could ships harvest plastic from the ocean -- and use it for fuel? (February 21, 2022).
* A step toward a practical thermoelectric converter: get the oxide out (October 11, 2021).
* Using the walls of a building as a rechargeable battery? (May 24, 2021).
* Solar sterilization of medical equipment (February 6, 2021).
* Growing on manganese ions as an energy source (September 15, 2020).
* Solar hydrogen -- with near 100% efficiency? (July 26, 2020).
* Solar cells: a new record for efficiency (May 26, 2020).
* Impact of shopping online on greenhouse gas emissions (March 28, 2020).
* A lithium-ion battery that might be good for a million miles? (October 29, 2019).
* Using ultrasound to recharge an implanted medical device (October 19, 2019).
* Why can't lithium metal batteries be recharged? (October 1, 2019).
* An air-conditioner you can wear? (August 19, 2019).
* Making hydrogen fuel from water -- from seawater (May 28, 2019).
* Progress toward making a homogeneous product from diverse lignins (May 17, 2019).
* Caffeine: is it good for solar cells? (May 13, 2019).
* Storing energy from an intermittent source -- as compressed air under the sea (March 3, 2019).
* Briefly noted... 2. The carbon-intensity of producing oil (November 14, 2018).
* MOST: A novel device for storing solar energy (November 13, 2018).
* A low-temperature battery (July 29, 2018).
* A path toward reduced global warming based primarily on improving energy efficiency? (July 24, 2018).
* Fracking and earthquakes: It's injection near the basement that matters (April 22, 2018).
* Manganese(I) -- and better batteries? (March 21, 2018).
* Wind energy: effect of climate change? (January 30, 2018).
* Making hydrocarbons -- with an enzyme that uses light energy (November 17, 2017).
* Making lithium-ion batteries more elastic (October 10, 2017).
* Solar energy: What if the Moon got in the way? (August 16, 2017).
* Using your sunglasses to generate electricity (August 14, 2017).
* Is solar energy a good idea, given the energy cost of making solar cells? (March 24, 2017).
* Hydraulic fracturing (fracking) and earthquakes: a direct connection (February 13, 2017).
* How much energy are we saving with energy-saving houses? (February 5, 2017).
* Capturing CO2 -- and converting it to stone (July 11, 2016).
* Los Angeles leaked -- big time! (April 29, 2016).
* Upgrading ethanol? (April 11, 2016).
* What happens when a lithium ion battery overheats? (February 19, 2016).
* A flow battery that uses polymers as the redox-active materials (January 8, 2016).
* Fracking: one scientist's perspective (January 5, 2016).
* Flow battery (January 4, 2016).
* A better way to oxidize americium? A step toward improved processing of nuclear reactor waste? (December 7, 2015).
* When the laundry piles up... An econophysics critique of smart meters (December 6, 2015).
* Making use of CO2 (November 10, 2015).
* Deaths from air pollution: a global view (October 23, 2015).
* More from the artificial forest with artificial trees (August 31, 2015).
* The energy landscape: looking ahead (August 16, 2015).
* The artificial trees in the artificial forest are now fixing CO2 (and making high-value products) -- naturally (May 13, 2015).
* A battery for bacteria: How bacteria store electrons (May 2, 2015).
* Turning lignin into a useful product (April 11, 2015).
* Boston is leaking (February 13, 2015).
* Space-based observation of atmospheric methane -- and the Four Corners methane hotspot (December 29, 2014).
* Butt batteries (December 16, 2014).
* Fracking: Implications for energy usage and for greenhouse gases (October 26, 2014).
* Impact of watching movies on global warming (September 30, 2014).
* Solar energy: A more efficient way to boil water? (September 12, 2014).
* Quality of oil and gas wells -- fracking and conventional (August 18, 2014).
* Supercapacitors in the form of stretchable fibers -- suitable for clothing (May 2, 2014).
* When lightning strikes a tree... (April 8, 2014).
* Methane leaks -- relevance to use of natural gas as a fuel (April 7, 2014).
* Windmills for your cell phone? (January 21, 2014).
* A simple way to make a supercapacitor with high energy storage? (January 6, 2014).
* Could vibration (or loud music) improve the performance of a solar cell? (December 11, 2013).
* A better way to divide 6 by 2: A more efficient way to use sugar (November 10, 2013). This post is also noted above in the section Alkenes.
* Shale gas recovery using hydraulic fracturing (fracking) (October 7, 2013).
* An artificial forest with artificial trees (June 7, 2013).
* What if your house could sweat when it got hot? (November 30, 2012).
* Engineering E coli bacteria to convert cellulose to biofuel (December 13, 2011).
* Energy wastage: The set-top box (August 1, 2011).
* Making electricity in your windows: sharing the solar spectrum (July 5, 2011).
* A Christmas present: Using concentrated sunlight to split water and CO2 (February 18, 2011).
* Planning (November 23, 2010).
* Cellulosics for energy: an update (October 30, 2010).
* Joint Center for Artificial Photosynthesis (JCAP) (August 16, 2010).
* Boiled batteries (July 19, 2010).
* Hydrogen fuel cell cars (June 8, 2010).
* Making biofuels from cellulose (May 17, 2010).
* Your waste body heat (May 4, 2010).
* MIT invents a better bicycle wheel (April 24, 2010).
* A new source of electricity (November 10, 2009).
* Sustainable Energy - without the hot air (September 16, 2009).
* Hydrogen cars? (June 24, 2009).
* Fast charging batteries (March 13, 2009).
* Materials for solar cells (March 10, 2009).
* Some fun reading: Fuel cell gadget and growing diesel (December 13, 2008).
* Converting Oklahoma to natural gas (October 1, 2008).
Musings home page (Current posts).
General resources...
Increase Your H2IQ -- an education page for the general public, from the Office of Energy Efficiency and Renewable Energy (EERE), US Department of Energy (DOE). Lots of information and resources. https://energy.gov/eere/fuelcells/increase-your-h2iq. (There is a link to the EERE home page at the top; among the other programs included at the EERE site: Biomass; Geothermal technologies; Solar energy technologies; Vehicle technologies; Wind and hydropower technologies.)
Biotechnology for Biofuels -- a new journal (2008). It is "an open access, peer-reviewed online journal featuring high-quality studies describing technological and operational advances in the production of biofuels from biomass". https://biotechnologyforbiofuels.biomedcentral.com/.
Biofuels page from the US DOE: https://genomicscience.energy.gov/biofuels/.
The Helios project, at Lawrence Berkeley Lab. Broadly, the goal of Helios is to develop new renewable energy sources, using both biological and chemical processes. https://www2.lbl.gov/LBL-Programs/helios-serc/index.html.
Articles... (2003-2008; most recent first)
Alternative energy for a sustainable future. A 2008 issue of Engineering and Science, the Caltech alumni magazine, was devoted to this topic. Good articles, written for the well-educated layman. Go to http://calteches.library.caltech.edu/710/, which is the table of contents for this issue, Volume LXXI, Number 2, 2008. Articles include "The Race for New Biofuels", by Frances H. Arnold; "Solar Fuels I: Rods and Stones", by Douglas L. Smith; "Solar Fuels II: The Quest for the Catalyst", by Harry B. Gray; "From Solar Fuel Back to Electricity", by Marcus Woo. Another good article from this magazine is listed below, under the title "Powering the planet".
There is great interest in making biofuels from cellulose rather than from starch. The disadvantage is the difficulty of breaking down cellulose. This difficulty is both inherent in the nature of cellulose and made worse by the presence of lignin. One approach to dealing with the latter is to develop trees with reduced lignin. Vincent Chiang, North Carolina State University, has developed trees with about half the lignin of natural trees. A news story on this work: Through Genetics, Tapping a Tree's Potential as a Source of Energy. November 20, 2007. https://web.archive.org/web/20120420025215/https://www.nytimes.com/2007/11/20/science/20tree.html. Link is now to Internet Archive.
S Cheng & B E Logan, Sustainable and efficient biohydrogen production via electrohydrogenesis. PNAS 104(47):18871-3, 11/20/07. They use a modified microbial fuel cell. Applying a small voltage allows electrons to be released in the form of hydrogen gas. Free online at: https://www.pnas.org/content/104/47/18871.
Energy at Berkeley. An article in California, the UC Berkeley magazine for alumni, introducing the range of energy work done here. Lisa Margonelli, Start-up U, with subtitle "With global warming breathing down our necks, energy is hot. And at Berkeley, green ideals are teaming up with that other green - money." September 2007. Free online at: https://alumni.berkeley.edu/california-magazine/september-october-2007-green-tech/start-u.
Water as fuel? Purdue Univ engineers, led by Dr Jerry Woodall, announced an improved process for generating hydrogen. The key chemical reaction is aluminum + water reacting to give hydrogen gas plus aluminum oxide. In the context of a car, the idea is that the car could easily carry aluminum and water, and generate the hydrogen gas as needed. The car would then run on burning the hydrogen. The new work is on the development of a new alloy of aluminum that allows practical generation of hydrogen. This is all quite reasonable. However, it has been misrepresented by some as using water as a fuel -- with the promise of a cheap and plentiful fuel. In fact, the aluminum is consumed in the process, as described above. The process depends on recycling the aluminum oxide product back to aluminum metal, a process done eletrolytically at considerable expense. It is this step that limits the economic merit of the process. Some have skipped over this point entirely, thus misrepresenting the potential of the process. The Purdue group is quite aware of this, and they discuss it in the item listed below. They offer a proposal which they think might adequately address it; I am skeptical of their proposal, but this is not the place for a detailed economic analysis. At least, the Purdue announcement presents not only an interesting technical development, but also the key economic hurdle it must overcome. Press release from Purdue: New process generates hydrogen from aluminum alloy to run engines, fuel cells. May 15, 2007. https://web.archive.org/web/20200503215428/https://news.uns.purdue.edu/x/2007a/070515WoodallHydrogen.html now archived).
Powering the planet. An article in the Caltech alumni magazine Engineering and Science Vol LXX #2, p 12, 2007. Based on a lecture, May 2007, by Caltech chemistry professor Nate Lewis. http://calteches.library.caltech.edu/4259/ Lewis emphasizes that the more important driving force behind developing new energy sources is the carbon problem, more than any immediate threat of running out of fossil fuels. Also see Lewis's web site at http://nsl.caltech.edu/home/. Try the sections on Research and on Energy. A series of more recent energy articles in this same magazine are listed above, under the title "Alternative energy for a sustainable future".
Rating fuels. Whether biofuels are or are not environmentally "better" than the usual fossil fuels depends on the details. Berkeley scientists have proposed a rating system so you can tell. The rating system would apply to specific processes, so that bio-ethanol would have different ratings depending on how it is produced. A UC Berkeley press release, "Green Biofuels Index would aid consumers, market". April 17, 2007. https://www.berkeley.edu/news/media/releases/2007/04/17_greenindex.shtml. The press release links to the full work.
Fossil fuels. A Nature web focus site on "fossil fuels and society", including the feature set listed under Further Reading (Ch 3) as Hall et al (2003), plus more.
https://www.nature.com/nature/focus/fossilfuels/index.html.
James K Hardy (University of Akron) has made available materials for a similar course. You can view his set of slides, by clicking on the chapter title. The site is now archived: https://web.archive.org/web/20140329064953/http://ull.chemistry.uakron.edu/genobc/.
Virtual Textbook of Organic Chemistry, from Dr W Reusch, Michigan State University. https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/intro1.htm. Excellent online textbook. Includes problem sets, and links to more. The book is for the common full-year basic organic chemistry course.
http://www.ochem4free.info/node/1. Organic Chemistry for free, an online intro organic book, by Richard & Sally Daley. This book is organized somewhat differently from most organic books. The primary organization is by reaction mechanism, rather than by functional group. Since we do little reaction mechanism in this course, this could be confusing at first. But for those going on with more organic, this book can be a useful complement.
Medical Biochemistry textbook, from Michael W King, Indiana University School of Medicine - Terre Haute. https://themedicalbiochemistrypage.org/. Also in Spanish.
Kimball's Biology Pages. An excellent biology glossary, plus lots of information. From Dr John Kimball, the biology textbook author retired from Harvard. In fact, the site is almost an online textbook in biology. https://www.biology-pages.info/. (This site is also listed for the Molecular Biology course, Ch 1 as a general source of background biology information, and it is listed under Biology: books and glossaries on the Internet resources: Biology - Miscellaneous page.)
Updated July 2022.
For IUPAC naming information for organic chemicals, check the following two sites.
1. https://iupac.qmul.ac.uk/BlueBook/
The new "Blue Book" -- Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. See bottom of the page for a pdf file.
2. https://www.acdlabs.com/resources/iupac-blue-book/
- ACD Labs has long hosted the IUPAC organic rules. Their page now just links to the new IUPAC Blue Book. But it also links to two older versions (1993, 1979). These may be useful for reference; in fact, the 1993 rules are still commonly used.
- Their ChemSketch program, which allows you to draw a structural formula and also to transform it into a 3D model, will name simple organic molecules. For more, see my ChemSketch page.
For more from IUPAC, see my page Internet resources: Chemistry - Miscellaneous under IUPAC.
http://www.organicworldwide.net. Organic Chemistry Resources Worldwide, a broad source of organic chemistry information.
| Some of the "General" sites listed here -- and many more -- are also listed on my page of Internet resources: Miscellaneous or Internet resources: Chemistry - Miscellaneous. Students are encouraged to browse that page, as well as Internet resources listed for other Chemistry and Molecular Biology classes. All of those are available from the List of pages of Internet resources. |
List of pages of Internet resources Organic/Biochem (X402) home page
Musings (newsletter -- current science)
Contact information Site home pageLast update: November 22, 2025