|
Introduction
Carbon atoms Carbocations Alcohols |
Amines
Protein structure Nucleic acids Summary Bottom of page; return links and contact information |
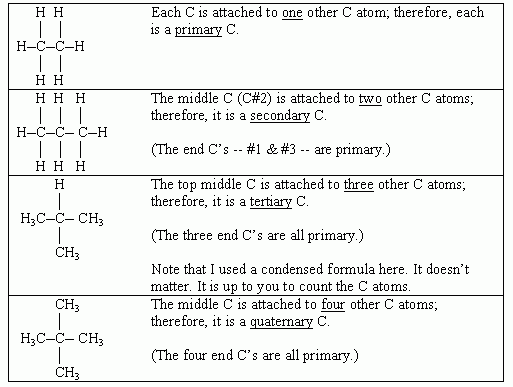
The following table illustrates this:

Ouellette 2/e, p 71.
Methane, CH4, is a special case. The C in methane is attached to zero other C. This C is sometimes called primary, for convenience. Alternatively, it can just be considered separate from any of these categories.
A carbocation (C atom carrying a positive charge) is classified the same way as a regular (neutral) carbon atom, above. That is, if the C of the carbocation is attached to one other C, then it is called a primary carbocation, etc.
The book discusses this in the context of addition reactions of alkenes; acid-catalyzed addition of HX proceeds through a carbocation intermediate. Ouellette 2/e p 120.
The same classification scheme is used for C atoms carrying a negative charge (carbanions) or carrying an unpaired electron (free radicals). Carbanions and carbon free radicals are not encountered in this course (though they are mentioned by Ouellette 2/e, in topics that we will not cover).
An alcohol is classified based on the C atom to which the -OH is attached. That is, if the alcohol -OH group is on a primary C, then the alcohol is a primary alcohol. Example: CH3CH2OH (ethanol) is a primary alcohol.
Hopefully, the classification of alcohols seems logical, following from the way C atoms are classified. But caution... the next time we use these terms is for amines, and that is a quite different story.
Ouellette 2/e p 219.
Alkyl halides are classified the same way as alcohols, discussed above. For example, CH3CH2Cl (chloroethane or ethyl chloride) is a primary alkyl halide, because the Cl is attached to a primary C. Ouellette 2/e briefly mentions this, p 204.
An amine is classified based on its N atom. If the N atom has 1 C attached, then the amine is primary, etc. Example: CH3NH2 (methylamine) is a primary amine, because the N has 1 C attached. The nature of the C itself is not relevant.
Note that the classification of amines is not done by the same general procedure as the others discussed above. Amines are not classified by their C atoms, but rather by the N atom.
The difference between how alcohols and amines are classified is made clear by comparing two compounds that look rather similar:
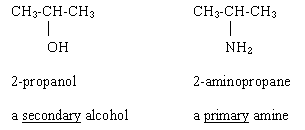
Ouellette 2/e p 291.
Amides and ammonium ions are classified the same way as amines. That is, groups based on amines are classified the same way as the amines.
Ouellette 2/e shows the classification of amides on pp 331 & 392.
Note that it is possible to have quaternary ammonium ions, but not quaternary amines. Ouellette 2/e introduces this, p 405. The tetramethylammonium ion is a quaternary ammonium ion. Choline, an important molecule in biochemistry, is also a quaternary ammonium ion; see Ouellette 2/e pp 349 & 377.
The same terms (primary, etc) are used to describe aspects of protein structure. These meanings have no relationship at all to the meanings above; they have no relationship to counting anything.
The term primary structure refers to the amino acid sequence in a protein.
The terms secondary and tertiary structure refer to the three-dimensional (3D) conformation of a protein chain. Secondary structure refers to the interactions of the backbone chain (that is, the amide linkages). Tertiary structure refers to interactions of the side chains. The distinction between secondary and tertiary is not entirely clear, and you will see various definitions. Emphasize understanding typical examples of secondary and tertiary structure, and let definitions emerge from those examples. Formal definitions are not so helpful here, unless you already understand the basic ideas.
Quaternary structure refers to the interaction between separate chains in a multi-chain protein. The types of interactions may be any of those found in secondary and tertiary structures.
Ouellette 2/e discusses the levels of protein structure in Sect 15.8.
The terms primary, etc., are used in essentially the same way for nucleic acids as for proteins, discussed in the previous section. Ouellette 2/e introduces this usage on p 452
The following table briefly summarizes all of the usages of the terms primary (etc) discussed above. These usages fall into three general categories. Terms shown in bold are section headings above.
|
Carbon atoms
Carbocations Carbanions Carbon free radicals Alcohols Alkyl halides | Classified by the C atom. |
|
Amines
Ammonium ions Amides | Classified by the N atom. |
|
Protein structure
Nucleic acid structure | Levels of structure |
Glossary Organic/Biochem (X402) home page Organic/Biochem (X402) Internet resources
Contact information Site home page
Last update: February 15, 2019