The content of this page is reasonably well covered in the Ouellette textbook, 2/e, Ch 5 especially Sections 5.2-3. Thus this page is now a secondary supplement, with more examples, for my Intro Organic/Biochem with Ouellette. Students in my class using Ouellette are responsible for this material at the level covered in the textbook and class materials; this page may be a useful supplement, but it is not essential.
This page was originally written for use with the Bettelheim textbook, 6/e. In Ch 14 Bettelheim presents aromatic compounds, but without a clear sense of what aromatic really means. This page was written to try to fill that gap. Textbook references below are to the Bettelheim book.
Bottom of page; return links and contact information
What is an "aromatic" compound? It is common to start by saying that aromatic compounds are compounds related to benzene. In fact, that is about as much as the Bettelheim textbook says about the nature of aromaticity. However, as you go on in organic chemistry you will find a variety of compounds called aromatic, even though they are not so obviously benzene derivatives.
Defining aromatic in terms of benzene is a useful start in an introductory course. As we will see here, it is not easy to give a more complete definition that is satisfying in an introductory course. But let's try...
First, why is aromaticity an issue? The special feature of benzene is that it is more stable than we might expect. If we write the structure of "1,3,5-cyclohexatriene", it looks like one of the Kekule structures for benzene. But benzene, a real chemical, does not have the properties we would expect for "1,3,5-cyclohexatriene". Why? Bettelheim discusses that benzene has two resonance structures, and that the actual structure is the resonance hybrid (6/e, pp 342-3). In general, resonance structures delocalize electrons, and thus stabilize a structure.
The resonance stabilization in benzene is considerably more than we might expect for simply having some double bonds near each other. Clearly, there is something special about benzene, which results in an unusual degree of resonance stabilization -- and results in the property called aromaticity. We can discuss this more, but doing so goes beyond course material. The purpose is to help those who are going on in organic chemistry get a sense of what this topic holds.
The resonance system in benzene involves six electrons. They occupy a series of p orbitals, one on each C atom. The p orbitals on neighboring C atoms overlap sideways, a type of bonding known as π (pi) bonding. Effectively, the six overlapping p orbitals form one large orbital -- a loop around the entire molecule. This "loop orbital" provides an unusual degree of stabilization, and is part of the secret to aromaticity. That is, one key feature of aromatic compounds is that there is a set of electrons in a loop orbital resulting from overlapping p orbitals around a ring. (Bettelheim refers to the "closed loop of six electrons", which he also calls the "aromatic sextet", 6/e, p 343.)
The paragraph above begins to talk about electron orbitals. There will be more about this below. There is also more about this on my page of Org/Bio Internet Resources in the sections Alkenes and Aromatics. Each of those sections includes a useful animation, from Tom Newton.
Let's look at some examples of compounds that are aromatic, but are less obviously "like benzene". As we discuss these examples, some patterns will emerge. Unfortunately, reasons for not discussing this too much in an introductory class will also emerge; understanding aromaticity even at an elementary level beyond "like benzene" requires understanding orbitals.
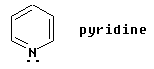
One simple example is pyridine, C5H5N, shown below:

| This example is simple enough, because it is very similar to benzene in structure. |
Some aromatic compounds with a heteroatom (an atom other than C or H) in the ring have a five-membered ring. These include pyrrole, C4H5N, and furan, C4H4O:
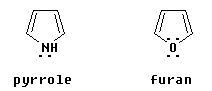
| What do these compounds share with benzene and pyridine that makes them aromatic? They appear to have only two double bonds, thus only four p orbital electrons perpendicular to the ring. But they also have one lone pair that is in a p orbital perpendicular to the ring. Thus they actually have six electrons that are in p orbitals perpendicular to the ring. |
It is important to analyze the orientation of any lone pairs. The lone pair shown in pyridine is not in a p orbital at all; rather, it is in an sp2 orbital in the plane of the ring. Thus these electrons are not able to be part of the loop of electrons that is characteristic of aromatic compounds. On the other hand, the N in pyrrole is an NH; remember that N always forms three bonds. The H is in the plane of the ring, and the lone pair that is shown is in a p orbital perpendicular to the plane.
For furan, one lone pair is in a p orbital perpendicular to the ring, thus part of the electron loop; the other lone pair is an sp2 orbital in the plane of the ring, thus is not part of the electron loop.
Ouellette 2/e Fig 5.3 is very good in showing the bonding and the unbonded p orbital electrons in pyridine, pyrrole and furan.
At this point, then, a pattern may be emerging: it seems that a loop of six electrons in overlapping p orbitals perpendicular to the molecular ring is a condition for aromaticity. Let's test this prediction by looking at some other structures.
Five membered rings of C. Look at cyclopentadiene, shown below. It has 2 double bonds, hence has 4 electrons in p orbitals perpendicular to the ring. But the other C is "saturated"; it is a -CH2- and is sp3-hybridized. No loop, not even 6 p electrons. Therefore not aromatic. Correct. But now look at the anion derived from this chemical -- the ion that would result if this chemical behaved as an acid, and gave off an H+; this ion is also shown below. Note that it now has six electrons in p orbitals perpendicular to the ring -- very similar to pyrrole, above. If pyrrole is aromatic, then maybe this ion should be? Yes, it is. A manifestation of this is that the parent compound, cyclopentadiene, is a rather "strong" acid -- by the standards of H attached to hydrocarbons. The acidity of cyclopentadiene is due to the stabilization of the resulting anion.

Ka for cyclopentadiene is about 10-16. That certainly isn't strong compared to compounds commonly discussed as acids, but it is 1029 times stronger than for the non-cyclic form of this molecule.
Now we have seen a variety of chemical species, both neutral molecules and ions, that are aromatic. They share the common feature that they all have 6 electrons in a continuous loop of overlapping p orbitals. Turns out that this is still not a sufficiently broad description of what makes a chemical aromatic.
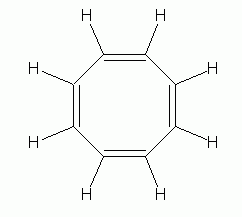
| An interesting example is 1,3,5,7-cyclooctatetraene (often simply called cyclooctatetraene), C8H8, shown at the left. At first glance, it would seem to be similar to benzene, except with a larger ring. But it is definitely not aromatic. Its chemical behavior is what you would expect for an alkene, and its shape is not planar, but is "tub-shaped", as shown below. |
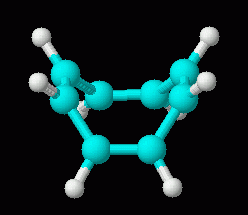
Now, the non-aromatic character of cyclooctatetraene alone is not too hard to explain. After all, if it were aromatic and planar, it would have bond angles of 135 deg. That is considerably more than the simple sp2 bond angle of 120 deg. So we might suggest that the bond angle strain of the planar form more than compensates for any gain due to aromaticity. However, there is more to the story. Turns out that it is fairly easy for cyclooctatetraene to gain 2 electrons, forming the dianion, C8H82-. This ion is planar, and is aromatic. Two drawings of this ion are below, followed by a 3D model.
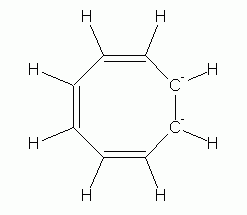
|
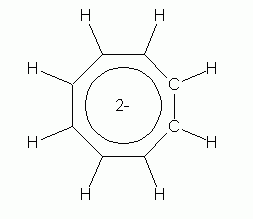
|
| cyclooctatetraene dianion | cyclooctatetraene dianion, shown with the circle that denotes an aromatic ring |
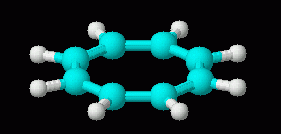
|

|
| cyclooctatetraene dianion, C8H82-, 3D | cyclooctatetraene, C8H8, 3D (neutral; repeated from above, for comparison of the 3D shapes of molecule and ion) |
So what do we learn from the story of cyclooctatetraene and its dianion? Prior to considering this, all our examples of aromaticity had six electrons in the π electron loop. Cyclooctatetraene has eight, and is not aromatic; its dianion has ten, and is aromatic. So clearly, six is not the only allowed number.
Study of many molecules and ions, as well as theoretical work that is well beyond our course, have indicated that a species will be aromatic if there are 4n+2 electrons in a planar π electron loop -- where n is any integer, starting with 0. (This is known as Hückel's rule.) 0? That would mean that a π electron loop with two electrons is aromatic. In fact, the cation derived from cyclopropene, shown below, is unusually stable, and is considered aromatic.
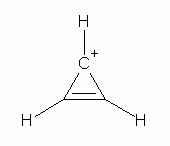
| The cyclopropenyl (or "cyclopropenium") cation, C3H3+. It has 4n+2 electrons in the π electron loop, where n=0; thus it satisfies Hückel's rule, and is aromatic. |
As another example, with a larger n, consider a particular isomer of 18-annulene -- the isomer with every third double bond cis. The following set of figures show three representations of its structure.
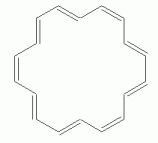
|
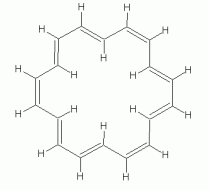
|
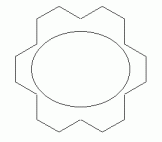
|
If 18-annulene is aromatic (n=4), we might expect it to be planar. In fact, some programs will calculate a planar structure for it. Actual measurement shows that is very slightly distorted from planar, due to the repulsion of the hydrogens that are inside the ring. The following figure shows 3D representations of both "forms" of 18-annulene; they have been rotated so you view them "edge on". The top structure is the planar form that one might naively expect; the other is the slightly distorted structure, which closely corresponds to what is actually observed.

(The original pdb files for these structures are from Dave Woodcock's collection at Okanagan: https://www.molecularmodels.ca/molecule/molecule_index.html. For more about pdb files and the Okanagan collection, see my page on the RasMol viewing program. This Figure was made using the Berkeley RasMol, which allows two molecules on the screen together. The display was set to "sticks", to make the planarity vs non-planarity more clear. Thanks to Greg for suggesting this view.)
The annulene name is used generically for cyclic molecules with alternating single and double bonds. The numeric prefix indicates the ring size. Benzene might be considered as 6-annulene, and cyclooctatetraene as 8-annulene. Note that all annulenes have the general formula CxHx (where x must be an even number), and that the term does not in itself imply aromatic character.
Aromatic compounds are more stable than we might expect when we see a structure showing single and double bonds.
We start our study of aromaticity with the classic case of benzene. But as we continue, we find examples of aromatic compounds that contain heteroatoms, charges, and rings of different sizes. As we explore these, we find that the key features of aromatic chemicals are:
Aromaticity requires a planar loop of electrons in overlapping p orbitals.
The number of electrons in the loop must be 4n+2, where n is an integer >= 0. We have not attempted to explain the reason for this requirement, but simply take it as empirical.
Most of the structures shown on this page were drawn with ISIS/Draw or ChemSketch. The 3D models for cyclooctatetraene were made with ChemSketch. The 3D structures for 18-annulene were modified from the RasMol files for planar and energy-minimized 18-annulene in Dr. Dave Woodcock's wonderful collection at University of British Columbia (Okanagan). The main address for his collection is https://www.molecularmodels.ca/molecule/molecule_index.html. I have posted guides to help you get started with ISIS/Draw, ChemSketch, and RasMol. (The ISIS/Draw program is no longer available.) These are all free programs, and easy to use and lots of fun! Thanks to Greg for assistance with developing this page.
A post in my Musings newsletter about aromaticity: Hückel at 40 -- that's n = 40: the largest known aromatic ring (February 1, 2020).
My page of Org/Bio Internet Resources has a section on Aromatic compounds, as already noted. It includes a more extensive list of related Musings posts.
| All feedback encouraged. Please e-mail me any comments/corrections/suggestions. See Contact information, below. |
Organic/Biochem (X402) home page Organic/Biochem (X402) Internet resources
Contact information Site home page
Last update: August 16, 2022