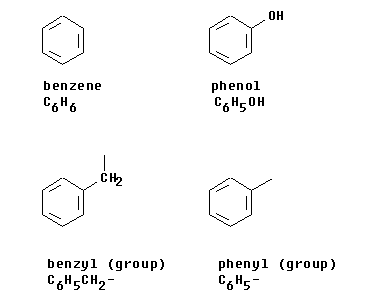
The top two structures in Figure 1 are molecules. The -ene suffix of benzene might indicate that it is similar to an alkene. The -ol suffix of phenol indicates that it has an -OH group.
Bottom of page; return links and contact information
The terms phenyl and phenol, along with benzene and benzyl, can confuse beginning organic chemistry students. Figure 1 shows what the four terms mean.
| Figure 1 --> |
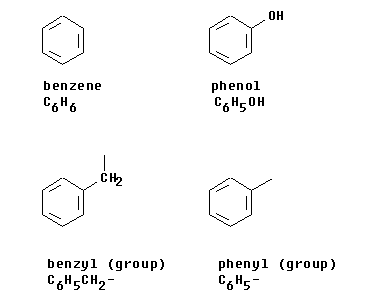
|
|
The top two structures in Figure 1 are molecules. The -ene suffix of benzene might indicate that it is similar to an alkene. The -ol suffix of phenol indicates that it has an -OH group. |
The lower two structures in Figure 1 show groups. The line extending off without anything connected is the line that shows this is a group, which should be attached to something. For example, one might have phenyl chloride (C6H5Cl, also called chlorobenzene) or one might have benzyl chloride (C6H5CH2Cl). (The structures of these two compounds are shown below in Figure 2.) The phenyl group is based simply on benzene, with one H removed. The benzyl group is based on methylbenzene (toluene), with one H removed from the methyl group.
| Figure 2 --> |
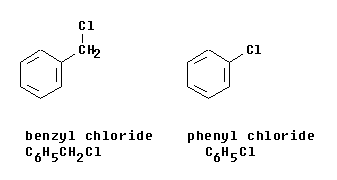
|
|
Figure 2 shows the molecules benzyl chloride and phenyl chloride; these are based on the groups discussed above. |
If you have the misfortune to come across the word benzol, be forewarned that this is an old German word for benzene. Similarly, toluol is a German word for toluene. Benzin is a German word for gasoline; it is related to the uncommon term benzine, used for some types of gasoline. (These German words are not in our books -- and you are not responsible for them.)
| All feedback encouraged. Please e-mail me any comments/corrections/suggestions. See Contact information, below. |
Glossary Organic/Biochem (X402) home page
Contact information Site home page
Last update: February 19, 2019