Ouellette 2/e Ch 12. However, this quiz broadly covers many topics from throughout the course.
Answer key
Bottom of page; return links and contact information
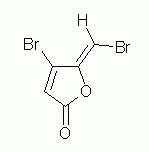
| 1. Consider the following compound (which is also discussed in the quiz on alkenes.) |

|
a. What functional group do the two O atoms, together, represent? There are two terms that are good here. One is general, one is more specific. Try to give both.
b. Draw the product of hydrolysis of the compound.
c. Name the hydrolysis product (part b).
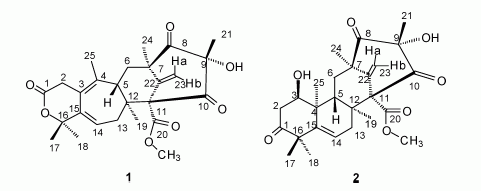
| 2. Consider the following two compounds: |
|
a. Identify all the ketone groups in these two compounds.
b. There are three other carbonyl groups that are not ketone groups. What functional group best describes these carbonyls?
c. For compound 2, which O-containing functional group is most likely to be oxidized by a mild oxidizing agent? What kind of a group is it? What would the oxidation product be?
d. For compound 2, how would you describe the remaining O atom(s) -- those not yet discussed in any of the parts above?
e. For compound 2, consider the possibility of an elimination reaction at the alcohol groups. Describe the product in this case. (Ignore any possibility of side reactions, etc.)
f. The compounds contain several carbon-carbon double bonds. For which of these do you need to specify cis/trans (or E/Z) in the name? Explain. (You need not make the assignments.)
g. (for fun) What originally caught my attention about these compounds is that they were given common names containing "berkeley". What is the relationship of these compounds to the University of California at Berkeley?
3. Look at the web page https://web.archive.org/web/20180417183410/http://stainsfile.info/StainsFile/dyes/insctdye.htm (now archived). It shows six chemicals that contribute to the color of insects -- and which have found commercial use as coloring agents. This question is about those six chemicals. All six are based on the same tricyclic structure, with various modifications; some have an additional group attached (at upper left).
a. What is the underlying tricyclic structure? For now, give it as a hydrocarbon.
b. Consider that underlying hydrocarbon (part a) plus the functional groups found on the middle ring.
* What is this chemical? (See p 141 for ring numbering.)
* What type of chemical is it?
* What is it closely related to (that is, what chemical would be easily oxidized to this chemical)?
(This question is largely on Ch 8 Section 9 of Ouellette.)
c. These chemicals contain two types of acidic groups. What are they?
d. Consider the carminic acid.
* What type of chemical is attached to the anthraquinone at upper left?
* What specific chemical is it?
* How is it attached to the anthraquinone?
e. Consider the side groups on all (four) of the chemicals that have them. Which one is the most acidic?
f. (Requires Ch 14, on N.) Consider the two N atoms. What functional groups do these represent? Include "degree". (Careful. You may have to draw out the structures carefully to see exactly how things are connected. The number of H is shown correctly.)
Answer key Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: April 14, 2019