Bottom of page; return links and contact information
1. a. ester (general term); lactone (term for a cyclic ester)
| b. Hydrolyze the ester to alcohol + carboxylic acid: |

|
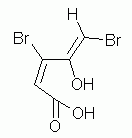
c. (E2,Z4)-3,5-dibromo-4-hydroxy-2,4-pentadienoic acid. (Putting the "2,4" just before "dien" is also good.)
Cis/trans? Hard to make it clear in this case. E/Z is better. And if you forgot to consider the configuration at the double bonds at all... It is something you need to become sensitive to. The name is incomplete -- and therefore wrong -- without it. Develop the habit of looking to see if double bond configuration needs to be specified.
2. a. Ketone groups are at carbons #8 and 10 of compound 1, and at carbons #1, 8, 10 of compound 2.
b. Both compounds have a carboxylic ester at carbon #20. Compound 1 also has an ester at carbon #1; this is a cyclic ester, and can also be referred to as a lactone.
c. Secondary alcohol at carbon #3 can be oxidized to a ketone.
d. Tertiary alcohol at carbon #9.
e. There are two -OH groups (parts c and d). Look at each separately.
* For the -OH at C #3, there is no H on the C to the right (C #4), but there is an H on the C to the left (C #2). Therefore, an elimination can occur to remove the -OH at C #3 and the H at C #2, giving a C=C double bond there.
* For the -OH at carbon #9, there is no H on the two adjacent ring C (#8, 10), but there is an H on the adjacent methyl group (C #21). Therefore, a double bond will be formed between C #9 and 21.
f. None of them. The double bond at carbon #22 is symmetric, with two H on one end. The other double bonds are asymmetric, but they are in small rings (6- or 7-membered). Double bonds in such small rings are always cis, and that information is rarely given in the name.
If you recognized that the ring double bonds are asymmetric and thus need to have their configuration specified, ok. It is basically convention to not specify it, because it is "so obvious" -- a reason that usually does not carry much weight in organic nomenclature. Note that calling these double bonds "cis" is a matter of convention, and assigning E/Z would take a fair amount of effort.
g. None. They are named after the Berkeley Pit Lake near Butte, Montana. This is an abandoned copper mine, and now a major Superfund site because of its acidic waters. The two compounds shown here, called berkeleydione and berkeleytrione (see part a), are made by a Penicillium fungus isolated from that acidic mine water.
This question is based on D B Stierle et al, Berkeleydione and berkeleytrione, new bioactive metabolites from an acid mine organism. Organic Letters 6(6):1049, 3/18/04. https://pubs.acs.org/doi/abs/10.1021/ol049852k. News story, including some follow-up: C Potera, Superfund site harbors extremophiles producing potent compounds. Microbe 1:506, 11/06. https://www.asmscience.org/content/journal/microbe/10.1128/microbe.1.506.1.
3. a. anthracene (p 141)
b. 9,10-anthraquinone. This quinone is the oxidation product of the corresponding di-phenol (p 237).
c. phenols; carboxylic acids
d. a sugar; D-glucose; it is attached by a C-glycosidic bond, α configuration.
e. The one on laccaic acid C; this one has a carboxylic acid group; the others have at most a phenol group.
f. Laccaic acid A: secondary amide. Laccaic acid C: primary amine.
The first one is actually a bit more complex. It is a carbamic acid, or rather the ester of one. A carbamic acid is a combination of amide and carboxylic acid on the same C. There is one shown on p 491 of Ouellette.
The quiz Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: August 22, 2019