| Figure 4b: |
|
This is a supplementary page, for A new approach to making large molecules, using a Vernier process (February 12, 2011).
Here is the starting figure, full size. (The figure shown on the main page is a reduced version of this.)
| Figure 4b: |

|
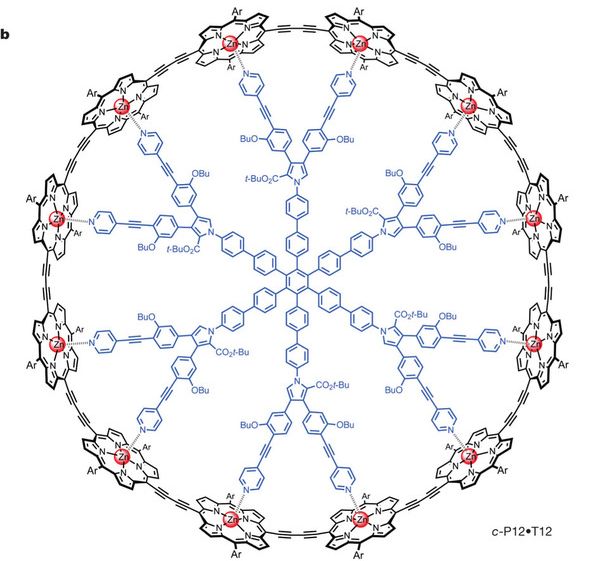
A single molecule? It may be better to think of it as two molecules: the big black ring around the edge, and the big blue inner part. In fact, the purpose of the work is to make the black outer ring molecule; the blue inner molecule is a template to guide making the outer ring molecule.
The following figure (4a) presents a diagram of the process, requiring no understanding of the chemical details.
| Figure 4a: |
|
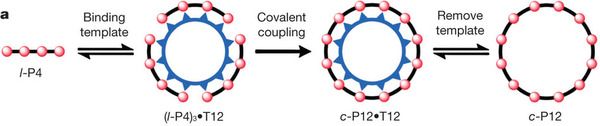
The key is to recognize the symmetry of the structure, as shown in the top figure (4b). The outer ring molecule consists of many -- 12 -- copies of the same piece -- which is mostly a flat black structure they call P. Similarly, the inner ring molecule contains many -- 12 -- copies of a complex structure. They call that inner complex structure T (for template); thus the complete inner molecule is simply T12: a ring of 12 copies of T. (We could also call it c-T12, where the c stands for circular.) A simple view is that we are going to assemble a ring of 12 P around the template of 12 T. Figure 4a shows how this works. They start by making a linear chain of 4 P: l-P4 (where the l stands for linear). Binding of l-P4 to T12 leads to three copies of l-P4 binding to each T12; the interaction of P and T is shown simply with circles and triangular points, with no chemical details here. In the third frame, they couple the P4 units together, to give a single circular P12 -- still bound to the template. Finally, they remove the template, giving the circular P12 molecule, which is what they wanted to make.
P stands for porphyrin. Porphyrin rings are interesting to chemists because they can conduct electricity. They are also of interest to biologists -- and therefore to each of us. A porphyrin ring is at the heart of each heme group, in the hemoglobin molecules that we use for carrying oxygen in our blood. Similar rings are also found in the chlorophyll molecules that collect light in plants and in vitamin B12.
Porphyrin rings commonly have a metal atom at the center: iron in our heme, magnesium in chlorophyll, cobalt in vitamin B12 -- and zinc in the P structures here. These metal atoms often interact with nitrogen atoms -- in the P rings, or nearby. Look carefully at Figure 4b above... you will see a key interaction between the P and T: the Zn of the porphyrin with an N of the template; this interaction is shown with a dashed bond.
So, are these figures (4a & 4b, above) what they accomplished in the new paper? Not really. Those figures show the old way to make c-P12. What they did was to improve on this. The huge structure in Fig 4b makes for a wonderful figure, but making that huge T12 template is quite difficult.
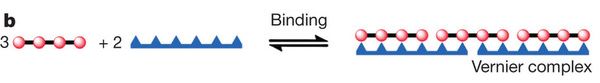
Instead of using that complex T12 template, what if we used a simpler T6 template? Can we make P12 on a T6 template? Hm, we could use two of them together. In fact, they specifically note that three of the P4 molecules could line up with two of the T6 templates, to effectively give P12 on T12. The following figure (Figure 1b) shows the idea, with a linear T6.
| Figure 1b: |
|
The complex is labeled a Vernier complex. What does that mean? Actually, it's easier to show than to explain. The figure shows how two structures with different numbers of units come together in a specific combination that makes them come out even. That's the Vernier idea. In this case, one structure has four units, the other has six. The least common multiple of 4 and 6 is 12; the figure shows how these two structures come together to produce a complex with 12 of each kind of unit.
Although the above figure shows the idea of a Vernier complex, it is not quite what we want. We want a circular P12. So, let's use a circular template -- a circular T6. Same idea, though the details turn out to be a bit more complex.
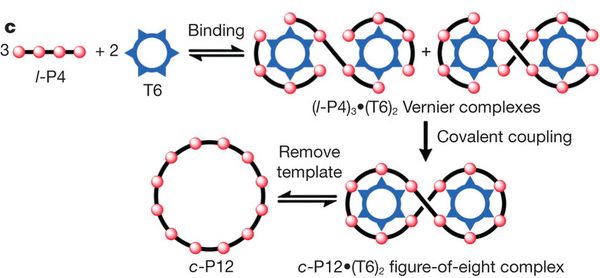
The next figure (Figure 1c) shows the idea with a circular T6. With the circular T6, there are a couple of ways to form the Vernier complex, but that doesn't matter for their purposes. In either case, they join the P4 units together and then remove the template. Those steps are just as before -- and so is the result: a P12 ring, that was assembled against a template. It works fine, they report -- and is easier, because of the simpler template.
| Figure 1c: |
|
Here is that new process, with the chemical structures:
| Figure 2a: |
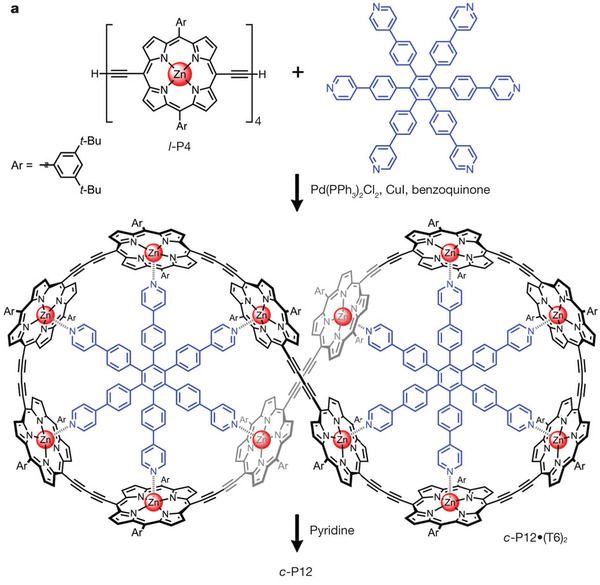
|
News story: Macromolecules from miniature templates. (Chemistry World, January 5, 2011.)
* News story accompanying the article: Supramolecular chemistry: Bigger and better synthesis. (C Hunter, Nature 469:39, January 6, 2011.)
* The article: Vernier templating and synthesis of a 12-porphyrin nano-ring. (M C O'Sullivan et al, Nature 469:72, January 6, 2011.) All of the figures in this post are from this article.
Return to this item on main page: A new approach to making large molecules, using a Vernier process (February 12, 2011). Or use your BACK button.
E-mail announcement of the new posts each week -- information and sign-up: e-mail announcements.
Contact information Site home page
Last update: May 13, 2021