Bottom of page; return links and contact information
1. and 2.

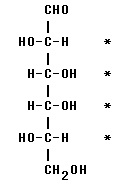
The stereocenters are C #2-5, marked with * in the structure above. You may recognize these as the usual stereocenters of aldohexoses. But you should also be able to figure them out. C1 is not chiral because it has a double bond. C6 is not chiral because it has 2 H. Each of the middle H has four different groups: an H, an OH, and two C-based units, which are different.
3. The -OH on the highest numbered stereocenter (the one nearest the bottom) is on the left side.
4.
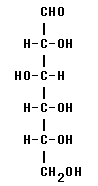
5. D-glucose
6. Glycosides are acetals. Start with the hemiacetal (cyclic) form of glucose, and substitute the ethyl group -- either "up" or "down" -- for the hemiacetal -OH group.
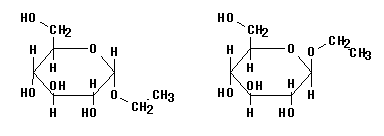
These are α-D-ethylglucoside (left) and β-D-ethylglucoside (right). You can also say glucopyranoside instead of just glucoside; that explicitly gives the ring size..
7. This gives you maltose. See Ouellette p 317 bottom for the structure.
The way the question was stated here, it does not matter whether the hemiacetal group of maltose (right hand end) is shown α or β. The book shows the β form, but the two forms would be in free equilibrium.
Note that this question and the previous question deal with the same basic issue, formation of an acetal from the hemiacetal form of glucose plus an alcohol. In this question, the alcohol is itself part of another sugar molecule, and the product is a disaccharide
8. Acid catalyst.
9. a. The O of the sugar -OH is missing; the attachment is C-C. This is called a C-glycoside.
b. α-D-glucose (or α-D-glucopyranose)
c. Carboxyl group and phenol groups. Carboxyl groups are more strongly acidic.
d. An anthraquinone is a quinone based on anthracene. Anthracene is the hydrocarbon with three aromatic rings side by side. Quinones are the oxidized form of di-phenols. (Ouellette pp 141 & 237.) (Many biological pigments are anthraquinones.)
e. The color of carminic acid is carmine (red). However, we should note that the color is pH dependent, because of all those ionizable groups. This pigment is commonly used at neutral pH, where it is red.
10. 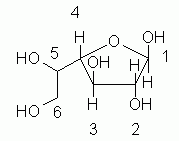
The key points for the basic part of the question:
* The hemiacetal is formed between the #1 and #4 carbons, to give the 5-ring.
* At C#1, the -OH is "up", since the question specified the β anomer.
11. ambidextrose (Thanks to Nick in Ontario, Canada, for contributing this question.)
The quiz Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: September 7, 2019