Ouellette 2/e Ch 11 & 6.
Answer key
Bottom of page; return links and contact information
The quiz is intended to be "closed book". However, you may look up sugar structures as needed to help you get started. For example, you will need to use the table of aldose sugars, Ouellette Fig 11.1. Such a table, and an example of a hemiacetal form, such as α-D-glucose, will be provided on the test.
1. Show the structure of L-galactose, using a Fischer projection.
2. Mark all the chiral centers in your structure in #1.
3. If you saw the structure that you wrote in #1, you should easily recognize that it is an "L-" compound. Why?
4. Draw the 2-epimer of D-mannose, using a Fischer projection.
5. What is the (common) name of the sugar that you drew in #4?
6. Draw the structures of the two glycosides that can be made from ethanol + glucose. Use Haworth projections. Name the two products.
7. Draw the structure of the α (alpha) glycoside that can be made using the 4-OH of one glucose + another glucose. Use Haworth projections or chairs, as you wish.
8. Consider the reactions shown in the previous two questions. What reaction conditions are needed for this type of reaction?
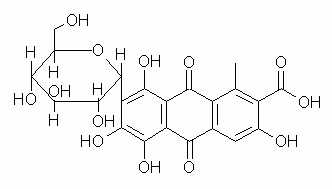
9. The Figure shows the structure of carminic acid, a useful pigment isolated from insects.

a. Carminic acid consists of a sugar attached to an anthraquinone. The attachment of the sugar is somewhat different than what we have seen so far. In what way?
b. What is the sugar? Since it is in the cyclic form, be sure to include the anomer designation.
c. The compound contains two types of acidic groups. What are they? Which is the stronger type? (X402 students: This requires, in part, the material in the chapter following carbohydrates.)
d. In part a, carminic acid was described as containing an anthraquinone. What is, in general, an anthraquinone? (X402 students: This is somewhat "advanced".)
e. ("for fun") What color is this pigment?
10. Draw the furanose form of an aldohexose; show the β (beta) anomer. Show the numbering of the C chain. For simplicity, you can ignore stereochemistry (other than showing the β anomer, as already noted). [Advanced: Do this for D-galactose. Following the stereochemistry may be difficult for many, but give it a try. It is ok to use models.]
11. On a lighter note... What ("common") name would you use to describe an imaginary sugar that has both left and right chirality?
Answer key Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: September 7, 2019