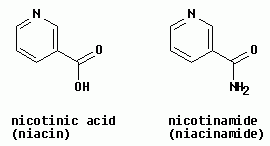
Bottom of page; return links and contact information
1. a. tertiary amine
b. ether
c. Some O functional groups involve an -OH group; these cannot possibly have the O heterocyclic, since O can only form two bonds. Similarly, groups with >C=O cannot have the O heterocyclic. Thus the only O groups that can have a heterocyclic O are those with the O attached to two C atoms. These include the ether, hemiacetal, acetal, ester and anhydride. If you are using the Ouellette book, all of these have been introduced by the time we get to amines.
Is it possible that the O might be in a ring but not between two C atoms? Yes in principle. No examples have come up yet, and they are not common. However, one good example, from biochemistry, is cyclic AMP. In this structure, two of the ring O are between C and P. The molecule is a cyclic phosphate ester. (In Ouellette: AMP, also called adenylic acid, is shown on p 451. The phosphate is esterified to the sugar at the 5' position. Now imagine that it is also esterified to the sugar at the 3' position. That gives cyclic AMP.)
The important part of this is the logic. Your list of suitable functional groups may vary, depending on how far you have gotten when you do this question. But your logic should be clear, for why you do or do not include specific groups.
d. Morphine has a phenol group, which has been replaced by an ether in codeine. Inspection of the structures shows -OH in morphine vs -OCH3 in codeine. [Calling the -OH group in the morphine an alcohol is not so good; simply calling it an -OH is evading the point. The -OCH3 in codeine is a methoxy group, but the general class is ether.]
e. The phenolic -OH is weakly acidic; the amino N is basic.
f. In acid, the N will be protonated, to produce the corresponding ammonium ion. In base, the phenol group will ionize, giving off H+ and leaving the phenolate ion.
Note that parts e and f deal with the same issues. If you answered one but not the other, you may not be properly connecting definitions to actual characteristics.
2. a. The one on the right. Aliphatic amines are stronger bases than aromatic amines.
There are two key steps in answering this. First, you need to recognize that the amine on the left is an aromatic amine, and the one on the right is aliphatic. Second, you then realize that aliphatic amines are stronger bases than aromatic amines.
| b. The structures are shown at the right. Both the "common" chemical names and the biochemical names are given. Indeed, the vitamin niacin is related to nicotine, by the reaction described in this question. However, that is not how it is made biologically. The biosynthesis of niacin is from the amino acid tryptophan. (Niacinamide is part of the hydrogen-carrying cofactor NAD, nicotinamide adenine dinucleotide. See Metabolism chapter. Also, both niacin and NAD(H) are mentioned in passing by Ouellette; see index.) |
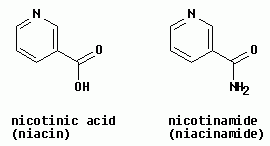
|
3. A 7-membered ring.
4. and 5.
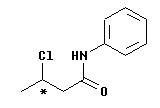
The one stereocenter is marked with an * (just below it). This C, C#3 of the numbered chain, has four different groups: Cl, H (not shown explicitly), and two C-based units. One of the C-based units has one C, the other is large.
The quiz Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: September 6, 2019