Ouellette 2/e Ch 14.
Answer key
Bottom of page; return links and contact information
The quiz is intended to be "closed book". However, you may need to look up some of the starting chemicals; use the Index. (If you simply read the answers from text material, you are missing the point of the questions.)
Some parts review earlier chapters.
For most parts of # 1-3, the general purpose is for you to explore features of complex organic chemicals, by looking at the structures. I would not expect you to know these structures, but you should be able to see features, as exemplified by the questions below. For most parts, the answer key does not contain drawings of these structures; let's try to communicate here using proper terminology.
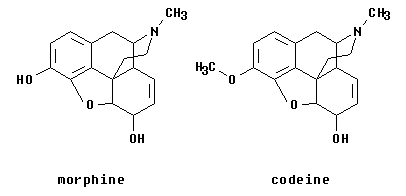
1. The figure below shows the structures of morphine and codeine.

a. What functional group does the N represent? And... what degree (primary etc)?
b. Consider the O atoms in the middle (bottom -- the heterocyclic O) of these structures. What functional group is this?
c. The previous part asks about a heterocyclic O -- what functional group this particular one represents. In general, are there any other functional groups that can involve a heterocyclic O? Which ones, if any? (Think about each O functional group you know.) Try to find some general principle that helps you decide.
d. Morphine and codeine differ at only one site. In terms of functional groups, how would you describe this difference? Give the general name of the functional groups, not just the atoms.
e. Identify any acidic or basic groups in morphine.
f. What will happen if morphine is put into an acidic solution? a basic solution?
|
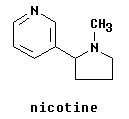
2. The following two parts are about nicotine; see structure at right. The two parts are independent.
a. Nicotine is a di-amine. If you add a little acid, which N will be protonated first? Explain how you can tell. b. Because aromatic rings are quite stable and the benzylic C is quite active, it is sometimes possible to oxidize off complex side chains, leaving only a carboxyl group. (Ouellette presented an example of such a reaction in Section 5.9; we skipped this.) Draw the product of such an oxidation of nicotine. Also draw the amide of that product. (Both of these compounds are of interest in biology, as discussed in the answer key.) |
|
3. Valium is a tranquilizer based on benzodiazepine. Look at the structure of either benzodiazepine or valium. What is unusual about one of the rings -- beyond one containing two N atoms (which is not all that unusual)? (Ouellette: see p 390).
4. Draw the structure of N-phenyl-3-chlorobutanamide.
5. Mark the stereocenter(s) in your structure for #4.
Answer key Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: September 6, 2019