Bottom of page; return links and contact information
1. a & b. Sarcosine is N-methylglycine.
c. Yes. It is a secondary amine. Only tertiary amines (with no H on the amino N) cannot form amides.
d. No. The resulting amide would not have an H on the N, thus could not donate to a H-bond.
[The common amino acid proline is also a secondary amine. Thus the issues for sarcosine, in parts c & d, are the same as for proline.]
2. a. HSCH2CH2CH(NH2)COOH
b. It is like cysteine, but has one more -CH2- group. [It is called homocysteine. The "homo" here means homolog.]
c. A lactone is a cyclic ester, so a thiolactone is ... ? Make the thiolactone by attack of the thiol S on the carboxyl group. Water is also made.
d. The thiolactone of part c has a five-membered ring. The thiolactone of the amino acid in part b would have a four-membered ring, which is quite strained.
So what happens to the homocysteine? Well, this question is attracting considerable attention, because of some evidence that homocysteine accumulation may correlate with heart disease. An article on this, and the relationship to dietary folic acid intake, is listed in the Ch 15 handout.
3. glutamic acid --> GABA + CO2. That is, decarboxylate the 1-carboxyl group of glutamic acid.
| 4. a. Hydrolyze the amide groups. |


|
| At pH = 7, all the amino groups and all the carboxyl groups are in the ionized form. | |
b. In these products, the amino group is on the #3 (or β) C atom; in the protein amino acids, the amino group is on the #2 (or α) C atom. Thus the original peptide given in the question is a β-peptide.
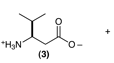
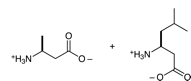
c. From the left: valine, alanine, leucine. That is, the first amino acid is the like valine, except that it has an extra -CH2- between the amino-bearing C and the carboxyl C; this is sometimes written β-hVal, where the hVal means homovaline, valine with an extra -CH2-. (Recall homocysteine, in another question, above.)
d. Enzymatic. The other common methods of hydrolysis use one or another extreme of pH.
e. They are amide N; amides are neutral functional groups.
This question is based on the article: B Geueke et al, A Novel beta-Peptidyl Aminopeptidase (BapA) from Strain 3-2W4 Cleaves Peptide Bonds of Synthetic beta-Tri- and beta-Dipeptides. J Bacteriol 187:5910, 9/05. https://jb.asm.org/content/187/17/5910. The structures shown are cut from their Fig 4.
5. Be sure to use the amino and carboxyl groups from the common part, not the side chains.
6. Label the H of the water as "δ+" and the O as "δ-". The polar group of the amino acid side chain should also be labeled to show its polarity. Then, show a weak interaction (dotted line, fairly long) between the Hδ+ of one molecule and the Oδ- or Nδ- of the other.
For #5 and 6, I encourage you to show me your answers. They involve fairly complex drawings, and for #6, many answers are possible. I can give you much better feedback by looking at your work than by offering answers here.
7. a. S
b. cysteine. In doing part a, you found that the amino group is top priority and the H is low priority. That leaves the carboxyl group and the side chain competing for priorities 2 & 3. For serine, in part a, the carboxyl group is higher priority than the side chain (OOO vs OHH on the first C). How could a side chain "beat" a C with OOO? By having an element above O on its first C. The only such example among the standard amino acids is cysteine. Thus, L-cysteine has an R stereocenter.
The discussion above makes a statement that is not entirely correct. It asks "How could a side chain "beat" a C with OOO?". And the given answer is "By having an element above O on its first C." Now, in the context of this particular question, that answer is fine. However, there is another way to beat the OOO of a carboxylic acid group. In fact, it uses a common group, which should be familiar to you from course material. What is it?
That cysteine is "odd" in its R/S designation is also noted on my page of Further reading: Old articles, in the discussion of the article by Leung (2000).
The quiz Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: September 27, 2019