Ouellette 2/e Ch 15.
Answer key
Bottom of page; return links and contact information
You need a table showing the standard amino acids (Ouellette Table 15.1). Except for that, the quiz should be "closed book".
Several of these questions are intended to help you see the relationship between structures of different chemicals.
1. Sarcosine is an unusual amino acid that occurs in nature occasionally. It is also the basis of a detergent, called Sarkosyl. Sarcosine is N-methylaminoethanoic acid.
a. Draw the structure of sarcosine.
b. What standard amino acid is closely related to sarcosine? How do they differ?
c. Could sarcosine form an ordinary peptide bond? Explain? (Consider the chemical structure. Don't worry about how this might happen biologically.)
d. If sarcosine did occur within a protein chain, could it form part of an ordinary α-helix? Explain.
2. One role of methionine in biological systems is to donate its S-methyl group to other chemicals.
a. What chemical is left after methionine donates its S-methyl group (and an H takes up the missing spot on the S)? Draw the structure.
b. What standard amino acid (other than methionine) is closely related to the structure from part a? How do they differ?
Check yourself for parts a and b before going on. You need the answers for a and b for the following parts.
c. Draw the thiolactone form of the amino acid made in part a. (If necessary, check your book to see what a lactone is.) What small molecule is a by-product of making the thiolactone?
d. Why is the amino acid made in part c more likely to form a thiolactone than the amino acid referred to in part b?
3. The chemical 4-aminobutanoic acid (also called γ-aminobutyric acid, or "GABA") is used as a neurotransmitter. It is made in one simple step from one of the standard amino acids. Which one, and how? Write a simple equation showing how one of the standard amino acids can be broken down into GABA plus a common small molecule. [This question may make more sense after you have studied the citric acid cycle, in the Metabolism chapter.]
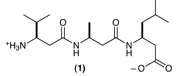
| 4. Consider the following chemical: |

|
a. The given compound is hydrolyzed, at pH = 7. Draw the products of hydrolysis. Show them in the proper form for pH = 7.
It may be good to check yourself on each part before going on to the next.
b. As a group, these products (from part a) are rather similar to the standard amino acids found in proteins -- except for one feature. What is the difference between these chemicals (the products from part a) and the standard amino acids?
c. Taking into account the difference noted in part b, the three products are otherwise just like which standard amino acids? (Look at the side chains -- the part attached to the amino-bearing C in each case.)
d. Part a of the question specified that the hydrolysis was at pH = 7. Of the various hydrolysis methods we discuss, which one is most likely here?
e. In the original structure, above, the N at the left is shown ionized. However, the other two N atoms are not. Why not?
5. Draw the complete structural formula of the following dipeptide:
N-terminus - glutamic acid - lysine - C-terminus
Take care to use the correct groups in forming the peptide bond.
6. Draw the structure of an amino acid with a polar side chain, and show how a water molecule could hydrogen bond to that side chain. Show the structure of the water molecule, and show enough detail so we can see what the attraction is between the water molecule and the amino acid side chain.
7. 19 of the 20 common amino acids are chiral; all 19 are of the L-series, with the α-amino group on the left in a standard Fischer projection. This question explores whether these amino acids are R or S, using the official IUPAC system for absolute configuration at stereocenters.
a. Is L-serine R or S? (Draw a Fischer projection, and figure it out, as you learned in the chapter on stereochemistry.)
b. Of the 19 chiral L-amino acids, 18 have the same R/S status, which you determined in part a. Which one amino acid is the exception? (This should be "fairly obvious" to you by scanning the table showing the amino acid structures. Think about how you assign R/S, and then see which amino acid will come out different. Of course, when you get a "hunch" by this process, check it by figuring out its R/S status, to be sure you are correct.) (Don't be distracted by some amino acids having a second stereocenter. That has no relevance here.)
Answer key Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: September 27, 2019