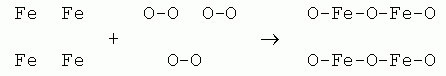
Bottom of page; return links and contact information
1. Of course, there may be many possible ways to interpret these analogies that are reasonably within the intended spirit. It is important, then, that you not just read the answers here as "correct", but that you think about why they are "logical". If you have other answers, tell me about them, with your reasons, and we can discuss.
a. Books. Libraries are "large", and are made up of books -- just as macroscopic chemicals are made up of the underlying atoms or molecules.
If you answered something like "chairs" (or tables), that would be a reasonable use of the particulate vs macroscopic distinction, but it does not reflect the special nature of "library", as distinct from any other kind of room or facility. Thus it seems to me that "books" focuses more on the specifics of this question. "Bookshelves" would seem to answer that objection. But isn't it "books" that makes a library what it is?
If you answered something like "atoms" (or molecules), again, that would be a reasonable use of the particulate vs macroscopic distinction, but again it does not reflect the special nature of "library". Atoms are perhaps the ultimate particles in any matter, from a chemistry viewpoint. If the focus is on chemicals, they may well be the relevant particulate view. But if the focus is on "library", then isn't "books" the more relevant or useful particulate view?
b. students
c. school
Parts b and c illustrate a broad idea, that there may be multiple levels at which we can analyze a system. In biology, populations contain individuals, the individuals have organs, and the organs have cells. In chemistry, matter contains molecules and atoms, and the atoms have particles such as protons and electrons. In physics, one may learn that the protons contain quarks -- a level of analysis we don't need to deal with in chemistry.
2. a. Chemical change. There are different chemical substances on the two sides of the reaction.
b. Physical change. The same chemical (free elemental iron) is present on both sides. Only the physical form of it has changed.
3. The first thing is to be clear which substance(s) we are talking about. The product side is 2 Fe2O3 (s). This is a compound, one chemical substance with a distinct composition, as shown by the formula.
In contrast, the reactant side, 4 Fe (s) + 3 O2 (g), is a mixture. There are two chemical substances here, iron and oxygen; each has its own distinct formula. The + sign between them is a clue there are two substances. One would not find a + sign in the formula of a compound.
4.
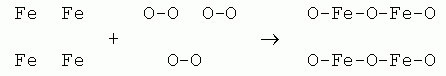
Drawing particulate diagrams is something of an art. Each diagram is intended to help you see key points. Which points are key depends on the question and context. Textbook diagrams may be very artistic, but being simple and clear is more important for us drawing them by hand. Here are some points to consider. If you think you have an alternative which is fine, or you just have a question, please check with me.
Symbols. This question is about specific chemicals. So why not use the elemental symbols. Using boxes and circles, or X and Y, just makes it less clear when specific chemicals are of interest.
Chemicals. The question is about specific chemicals and a chemical change. Thus showing the correct chemicals is important. The question says O2. Showing O-O makes O2 clear. (OO is also ok.) But showing 2 O2 as O O O O would not be; it does not show the given chemical, O2, even though it does show the correct number of O atoms.
Number of atoms. A key point in this question is showing that atoms are conserved but that they move around, to form new chemicals. Thus it is important that you have the same number of each kind of atom on each side. You will build on this idea later, when you formally learn about balancing chemical equations.
The answer above starts with 4 Fe atoms? Is it ok to start with some other number of Fe atoms? Perhaps. But the question has an equation that starts with 4 Fe, so why not start with 4 Fe? If you start with 8, you will have to show twice as much of each chemical. That's ok, but certainly does not enhance the answer. If you start with 2 Fe, you will find that you cannot make it work.
Organizing the symbols (atoms) within one chemical. At this point, it doesn't matter; the main purpose here is counting the atoms. Whether you write O2 horizontally or vertically is irrelevant - so long as you show that the basic unit is two O together somehow. Whether you write Fe2O3 as O-Fe-O-Fe-O or as Fe-Fe-O-O-O is not relevant at this point.
Physical state (phase). Since this question is about a chemical change, I suggest you not try to show the physical state here.
5. The following sketch is an example of how one might show this. But a big caution up front: showing phases, especially liquids, clearly in particulate diagrams is tricky. Most important is to try to show the important differences between the phases. And one of the most important differences is not easily shown in a simple diagram.
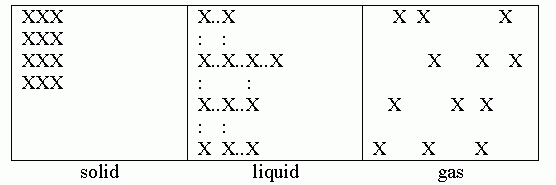
The particulate diagrams for solid and gas are fairly straightforward. A solid consists of a group of particles together in a fixed arrangement. So we show them together, in an orderly arrangement. A gas consists of particles that are independent of each other, so we show them "far" apart. But the key characteristic of a liquid is that the particles are moving around even though close together. It is hard to capture that feature -- movement -- in a static diagram. (Of course, particles in a gas are also flying around, but we still capture the essence of a gas by simply showing the particles far apart.) If we show the particles for a liquid far apart, that is not really correct, and makes it look like a gas. If we show them together, it may tend to look like a solid. What I did in this figure was to show them together, but in a somewhat irregular arrangement. By showing the bonds (which I omitted for the solid), I showed that the bonding is "partial" and irregular.
More comments...
Shouldn't the gas molecules be further apart than shown in the sketch above? Perhaps. (How far apart they should be depends on the temperature.) But that is hard to show to scale in a small diagram. The question here asked you to show 12 atoms. If you spaced them "more reasonably", the sketch would be quite large. Use judgment. Show the main features of relevance. If the question asked about spacing, then consider that. The sketch above shows that the particles in the gas are not bonded with each other.
Since the "liquid" sketch above shows bonds, shouldn't the "solid" sketch also show the bonds? Good point. If you want to do that, fine. I chose not to here, and dealt with that in my comments.
The question said to use "any element". But aren't some elements normally diatomic, e.g., N2? Yes, good point. But the diagram above shows the relationship between particles in three phases. It doesn't matter what the particles are. If the substance is N2, then each X represents one N2 molecule.
The point to emphasize is that there is no single "right" way to draw these diagrams. You draw them to make the points that are relevant. I encourage students to make a short note by any diagram that they think might not be entirely clear. And if your instructor likes such diagrams a particular way, by all means follow your instructor's guidelines -- but also try to understand the basis of them. Feel free to show me your sketches here, and we can discuss their strengths and weaknesses.
The quiz Quiz list Intro Chem (X11) home page
Contact information Site home page
Last update: August 29, 2019