Bottom of page; return links and contact information
1. a. HNO3 (aq) + F- (aq) --> HF (aq) + NO3- (aq)
Proton transfer reaction: transfer H+.
When balancing equations that include ions, be sure that the charges are the same on both sides. In this case, the total charge is -1 on each side.
b. From left to right... acid, base, acid, base. HNO3 and NO3- are a conjugate acid-base pair; they differ only by one H+. Similarly, F- and HF are a conjugate pair.
c. Nitric acid is stronger. It is one of the common strong acids. Most acids are weak.
2. a. H2+ + H2 --> H3+ + H
You might think of this as
H-H+ + H-H --> H-H-H+ + H.
But when you get that far, it is better to re-write the equation using the usual formulas, as above.
b. From left to right... acid, base, acid, base. H2+ and H are a conjugate acid-base pair; they differ only by one H+. Similarly, H2 and H3+ are a conjugate pair.
Conceptually, this question is almost identical to the first question. If you could do #1, you should be able to do this one. The difference is that #1 involves familiar chemicals, and you should be able to guess or recognize what is happening. In contrast, #2 involves unfamiliar chemicals; however, you are told what the reaction is. You are told that H2+ is the acid. Acids give off H+. So you know that H2+ gives off H+ -- which leaves H. Where does the H+ go? Well, the question says that the other reactant is H2. So H2 must get the H+ (must be the base); that makes H3+.
This question is taken from an article: R L Hudson, Astrochemistry examples in the classroom. J Chem Educ 83:1611, 11/06. The general spirit of the article is to explore some non-earth chemistry. The principles are often the same, but with "unusual" chemicals -- as this question illustrates. Oh, the phases for part a? Well, gas; interstellar space. (The paper is at https://pubs.acs.org/doi/abs/10.1021/ed083p1611. There may also be a copy freely available; put the article title in Google Scholar, and check the listings.)
3. To calculate pH from [OH-] requires two steps. First calculate [H+], then calculate pH.
[H+] = Kw/[OH-] = 10-14 M2/5.71x10-3 M = 1.75x10-12 M.
pH = -log[H+] = -log(1.75x10-12) = 11.76
Note that the M is dropped when taking the log. For those who are bothered with this step, there is a more complex explanation.
Significant figures. Exponents are exact, and do not count when determining SF. When you take a log, the exponent becomes the part before the decimal place. Thus pH 11.76 is 2 SF. The 11 represents the exponent part; only the 76 count as SF.
You could also first calculate pOH, then pH.
4. List i is best. Nitric acid is a strong acid; that should be common knowledge, but it is also stated in the question. Saying that something is a strong acid (or a strong electrolyte) means that it is fully ionized in solution.
List ii shows the behavior of a weak acid, which is partially ionized. List iii shows the behavior of something that does not ionize at all -- a non-electrolyte, such as alcohol or sugar.
Lists i, ii & iii are about the only possibilities there are. Of course, one could vary the fraction shown as ionized, but that is really missing the point. The idea is that an acid (or electrolyte) is either fully (or nearly fully), partially, or not ionized. So you should not need option iv. If you think you do need it, please check with me, and tell me why you think it is different.
5. There are two general ways one can tell which solution is more acidic. One is to have the [H]+ of both solutions; the one with the higher [H]+ is more acidic. The other is to have the pH of both solutions; the one with the lower pH is the more acidic. These two approaches are equivalent, since high [H]+ is equivalent to low pH. In my answers below, I will use one approach in each part. It is fine if you use the other.
(I suppose you could also have the [OH]- of both solutions; the one with the lower [OH]- is more acidic -- because it has higher [H]+. That would generally seem to be a more convoluted way to approach these, but it is fine if it seems appropriate to you.)
a. The second solution, pH = 10.1, is more acidic. No calculation needed; just compare the two given pH values; the lower pH is more acidic.
b. The two solutions are described in different ways. You need to convert one of them so it is described the same way as the other one; then you can compare them. The second solution is pH 2 (-log 10-2), so it is more acidic.
c. This part is exactly like the previous part, except that the pH values are non-integers. Therefore, you will need to use your calculator. The pH of the second solution is 3.62. Thus, the second solution has the lower pH, and is more acidic.
d. The second solution has [H+] = 10-1 M (by using Kw). Thus the second solution has higher [H+], and is more acidic.
e. The second solution has [H+] = 10-5 M (by using Kw), and pH 5. Thus the second solution has lower pH, and is more acidic.
f. You have two solutions of acids at the same concentration. So the conceptual point is that the one with the stronger acid will be more acidic (have more [H+]). How do you know which is stronger? Well, in the general case, you might have to look them up. But that is not allowed here, and should not be necessary. One of the acids here -- HCl -- is one of the few common strong acids; propanoic acid is not one of them. Even if you do not know what it is, it is a very good "guess" (using good chem logic) that it is weaker than HCl.
Emphasize that there are two levels of the answer here. The first is conceptual: understanding what the question means, and that the "real issue" here is "which of these two acids is the stronger acid?" If you got that far, good. I'd give good partial credit to a student who answered that much. The second level is knowing, or figuring out, which acid is stronger. How that plays out will vary for different classes. What I wrote above works for my classes, where I ask students to know a short list of common strong acids -- and to then assume that other acids are weak.
So what is propanoic acid? It is perhaps best known as the odor of Swiss cheese. Propanoic acid (also known as propionic acid) is very similar to ethanoic acid (= acetic acid, in vinegar). Propanoic acid has three carbon atoms (as in propane), whereas ethanoic acid has two (as in ethane). The structure of propanoic acid can be shown, in a condensed form, as CH3CH2COOH.
g. The second solution is more acidic. Lower pH is more acidic.
Don't let the negative pH distract you. pH = -1 means that [H+] is 10-(-1) = 101 = 10 M. There are some complications of detail at such high concentration, but the basic idea is fine. The pH scale is fundamentally open-ended.
6. a. First, we need to be sure we have a clear criterion of what we mean by "the solution is acidic". For simplicity, I will take it to mean pH < 7; that is, [H+] > [OH-]. Some instructors may prefer some other cutoff, as a practical matter. That's fine. Just be sure that you have some specific cutoff. If you use a cutoff other than mine, you will need to adjust my answer here to fit your cutoff, but that is probably a minor concern here. (I'd be somewhat surprised -- and suspicious -- if you choose a criterion that is different enough to affect the answers I give.)
By that criterion, the following solutions are acidic: both in part b; both in part c; both in part d; second solution in part e; both in part f; both in part g.
Comparison of this question with the previous one makes an important point about terminology. We use the term "acidic" in two related but distinct ways. If we consider two solutions, one each at pH 10 and 11, the pH 10 solution is "more acidic" than the pH 11 solution: it has a 10-fold higher concentration of hydrogen ions. But neither solution is "acidic" -- a comparison against an "absolute standard", where "pH < 7" means acidic.
Both terminologies are proper, and they really are quite clear. Be sure you understand whether the term is being used in an absolute sense or a comparative sense.
The term "strong", which we have used in some of these questions, also has a similar dual meaning. There is a small group of acids (such as HCl) that are commonly referred to as "strong", reflecting that they are nearly full ionized. However, we can compare any two acids and ask which of them is "stronger": more ionized. For example, neither acetic acid nor hydrocyanic acid (hydrogen cyanide) is a strong acid. However, comparatively, acetic acid is stronger than hydrocyanic acid; at a given concentration, acetic acid is more ionized than hydrocyanic acid.
Another example is the term "concentrated". If we have two solutions of NaCl, one each at 0.001 M and 0.01 M, the second is "more concentrated" than the first: it has a 10-fold higher concentration of NaCl than the first. However, neither solution would likely be considered "concentrated" by comparison against any absolute standard.
b. The value of Kw depends on T. The commonly given value, 10-14, is for 25° C. In parts d & e of the previous question, you needed to use Kw, so you made use of the temperature info.
The effect of T on Kw is not very big over the range of T that we most often use. Some instructors may not even bother to mention the T effect, but if you look carefully, you will most likely find that it is mentioned, even if not emphasized, in your book. It is something to be aware of, and becomes important if working far from 25° C.
To give some feel for how Kw varies... pKw is 14.94 at 0° C, 14.00 at 25° C, and 13.26 at 50° C. At 100° C, pKw = 12.26; thus the pH of pure, neutral water at 100° C is about 6.1. Under extreme T and P, pKw varies widely. For example, at 1000° C, pKw is 24.93 at 250 atmospheres pressure and 7.85 at 10,000 atmospheres.
7. a. HCl (aq) + NaOH (aq) --> NaCl (aq) + H2O (l)
b. The HCl; the titration takes less volume of the HCl, so it must be more concentrated. (According to the balanced equation, part a, the number of moles must be equal for the NaOH and HCl.)
c. To calculate moles HCl, use the volume and concentration of the HCl.
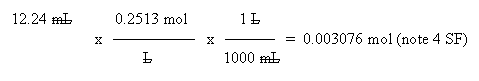
d. From the balanced equation, you know that the number of moles is equal for the HCl and NaOH. Thus the number of moles of NaOH is also 0.003076. The vol is given. So just calculate the molarity (mol solute per liter solution):
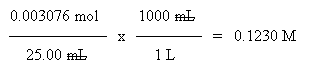
Check... The calculated result in part d shows that the concentration of the NaOH is indeed less than the concentration of the HCl, in agreement with the prediction in part b.
The quiz Quiz list Intro Chem (X11) home page
Contact information Site home page
Last update: August 28, 2019