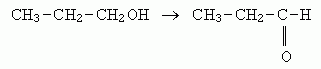
Bottom of page; return links and contact information
|
1. a. butane (n-butane)
2. a. alkane; none. (Alkanes contain only C-C and C-H single bonds; there is no "functional group" in the sense that emerges later.)
3. a. all of them
4. a & f. none.
5. a. 1-butene --> butane
6. 1-butene --> 2-butanol. In this case, the major product is determined by Markovnikov's rule. |
7. 1-propanol --> propanal (the aldehyde).
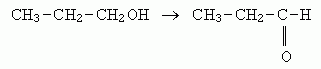
[The aldehyde could be easily oxidized further to the acid (propanoic acid), but the question specified to show only the first step].
Officially, you are not responsible for naming aldehydes and ketones (which appear in the answers to this question and the next one) as of Ch 9. However, those names are officially in Ch 10.
8. a. 2-propanol --> propanone (acetone), by mild oxidation.
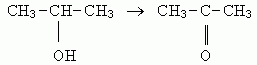
b. 1-butene --> 2-butanol --> 2-butanone. The first step was discussed above, in #6; the second step is the same kind of reaction as in #8a.
9. t-butanol --> 2-methylpropene (isobutene)
10. a. none
b. phenols and thiols.
(The acidity of phenols is very dependent on what other groups are attached to the ring. Phenol itself is a quite weak acid; 2,4,6-trinitrophenol is a fairly strong acid, because of all the electron-withdrawing groups.)
11. a. alcohol > ether > hydrocarbon. Hydrocarbons are nonpolar, with weak intermolecular interactions; thus they have lowest BP. Ethers are polar, because of the O, but cannot hydrogen bond with other molecules of themselves, because they lack a polar H. Alcohols are not only polar but can also hydrogen bond with other molecules of themselves; thus alcohols have the highest BP.
b. alcohol = ether > hydrocarbon. Both alcohols and ethers can interact with the solvent water by hydrogen bonding.
c. A large hydrocarbon might have a higher boiling point than a small alcohol. High mass is the other major factor that tends to increase BP. (I am not very concerned about whether your choice of a specific hydrocarbon happens to be a good one here, so long as your logical point is correct. The smallest alcohol is methanol, with BP = 65° C. The boiling point of hexane is 69° C.)
d. No. In part c, we achieved a higher BP by increasing the size of the molecule, even though it is entirely nonpolar. That change would probably reduce the solubility.
12. a. ether
b. the triple bonds (alkyne groups); addition reaction.
The Figure on the quiz page is part of Fig 2 from: C J Hawker & K L Wooley, The convergence of synthetic organic and polymer chemistries. Science 309:1200, 8/19/05. Online: https://www.science.org/doi/abs/10.1126/science.1109778. Indeed, the reaction shown in that figure is an addition across the triple bonds -- all 12 of them.
The quiz Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: March 2, 2022