Bottom of page; return links and contact information
1. Palmitic acid is a lipid. The difference between the two compounds is the length of the C chain: 3 vs 16. With the long alkyl chain, the hydrophobic end is more or less independent of the hydrophilic end. With the short chain, it is unreasonable that the two ends can dissolve independently. Actually, it should occur to you that propionic acid is likely to be soluble in water. You know from common experience that ethanoic acid (in vinegar) and 2-propanol (in rubbing alcohol) are soluble in water.
2. Less dense. You should know that lipids are not very dense. You may know this from common experience, that fatty materials tend to float on water. Further, you know that alkanes are less dense than water, and lipids are very much like alkanes.
3. Both of these are examples of double replacement reactions, a common type of reaction from general chemistry. Part a is an example of the type covered in general chemistry. Part b is exactly the same idea, but involving organic chemicals. Zinc undecylenate is an antifungal agent found in many over-the-counter products.
a. Ba(NO3)2 (aq) + Na2S (aq) --> 2 NaNO3 (aq) + BaS (s)
b. Zn(NO3)2 (aq) + 2 NaUnd (aq) --> 2 NaNO3 (aq) + Zn(Und)2 (s)
Some variations of those equations are acceptable. If you have a variation and would like feedback on it, check with me.
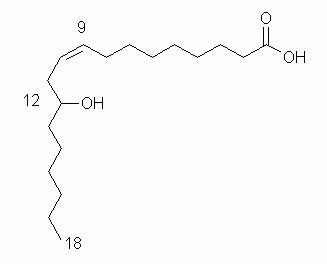
| 4. a. |

| Be sure that you have the correct chain lengths, things at the right positions, and the correct configuration at the double bond. |
b. There is a stereocenter, at C-12 (with the -OH group); the stereochemistry at that position is not specified in the name I gave.
If you think you see another ambiguity, please check with me.
The stereocenter is R. For those who want a bit more here, take this additional information, rank the four groups at C-12, and draw the compound showing the configuration at the stereocenter. Feel free to check this with me; checking the ranking is fairly easy; a simple text message is sufficient.
c. cis
d. There is no rule that Z = cis. Indeed, that is probably most common, but one really needs to check. In this case, at each relevant C, there is an H and a C. C > H, and the two higher priority groups are on same side = zusammen (together) = Z.
e. 12-hydroxyoleic acid. All the information about the double bond (position and configuration) is conveyed by the common name "oleic acid".
f. 12-hydroxyoctadecanoic acid or 12-hydroxystearic acid.
g. 12-hydroxystearic acid + LiOH --> lithium 12-hydroxystearate + water. Remember a general idea from chemistry: acid + base --> salt + water; this is a perfect place to use that.
h. The difference is the hydroxyl group -- absent in oleic acid, but present in the given compound. Oleic acid is a normal and common constituent of membranes. But that -OH disrupts the non-polar character of the fatty acid side chain, so makes the hydroxy compound unlikely to occur in normal membranes.
This question is based on an article: C J Donahue, Lubricating grease: A chemical primer. J Chem Educ 83:862, 6/06. Online: https://pubs.acs.org/doi/abs/10.1021/ed083p862. Among other things, the article describes how (Z)-12-hydroxy-9-octadecenoic acid -- also called ricinoleic acid, and a major ingredient of castor oil -- is converted into lithium 12-hydroxystearate, which is an important constituent of some lubricating greases.
The last part brought up the issue of whether the compound, which we can now call ricinoleic acid, is likely to be found in ordinary membranes. Note that it is not found there; it is found in the oil (storage fat), as triglycerides.
5. The following figure shows structures for several parts of this question. The parts of the Fig are referred to below.
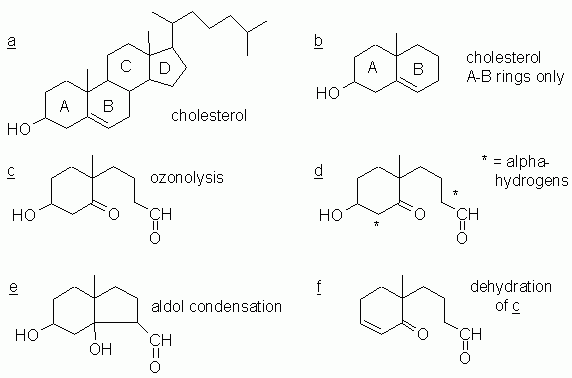
a. The A and B rings. These are the only two rings that have reactive functional groups in them. Part a of the Figure, above, shows the complete structure of cholesterol. Part b shows the AB rings; we will use this smaller structure as the basis of the following parts.
b. See part c of the Figure. Ozonolysis cleaves a double bond, and leaves a carbonyl group at each C of the double bond. In this case, that means we get one aldehyde and one ketone.
c. None. Your basis for answering this should be the reactions you have come across so far in this course (and this book). But it seems to be true that the product shown above is characteristic of ozone oxidation of an alkene. We will return to this point below.
d. Acidic α hydrogens are available on two carbons, one adjacent to each of the carbonyl groups. These α C that have acidic H are marked with * in part d of the Figure. (Each of those marked α C has two hydrogens.)
There are two plausible intramolecular aldol condensation reactions. Each would involve one of the α C (with an acidic H) and the other carbonyl carbon. One of those reactions would give a five-membered ring, and the other would give a seven-membered ring. Remember that 5- and 6-rings are generally the most common. Using that as a reason gives the aldol product shown in part e of the Figure.
e. Part f of the Figure shows the dehydration product that was observed. The other possible dehydration product would have the double bond one position "higher" in the structure.
Why is this an interesting series of reactions? This series of questions is based on an article: P Wentworth, Jr., et al, Evidence for ozone formation in human atherosclerotic arteries. Science 302:1053, 11/7/03. Online: https://doi.org/10.1126/science.1089525. There is an accompanying news story: J Marx, Medicine: Ozone may be secret ingredient in plaques' inflammatory stew. Science 302:965, 11/7/03. Online: https://doi.org/10.1126/science.302.5647.965a. The essence of the story is that they find the ozonolysis product of cholesterol (part b of the question, above) in real arterial plaques. Because that particular structure is only made by ozone (part c), they infer that ozone is made in the arteries. (And they have other circumstantial evidence to support that.) The other compounds discussed above come up during their characterization of the material.
This article has gotten a lot of attention because of its obvious implications for heart disease. However, be cautious in interpreting it. The relationship between the observed compound and disease processes is speculative. Further, it is not clear whether exogenous ozone (e.g., air pollution) could cause such plaque material. I am sure that we will be hearing more about this story.
The basic claim that ozone is made in the neutrophils has been much challenged. For example, see the article: A J Kettle & C C Winterbourn, Do neutrophils produce ozone? An appraisal of current evidence. BioFactors 24:41, 2005. Online: https://iubmb.onlinelibrary.wiley.com/doi/abs/10.1002/biof.5520240105. (This article is listed along with Marx in the chapter handout.) I'm inclined to say that the story has not been resolved.
The quiz Quiz list Organic/Biochem (X402) home page
Contact information Site home page
Last update: April 21, 2022